Page 232 - Battery Reference Book
P. 232
18/16 Lead-acid secondary batteries
D Stationary cells (Exide), cycled, this stabilizing effect means less sensitivity to
pasted plates, PbCa alloy occasional deep discharges.
1
o OPZS stationary cells,
200 -
-c tubular plates, 1.6% Sb,
U selenium 18.5 Separators for lead-acid
8 automotive batteries
2 100 e Typical properties for separators for antimonial lead
a @ a p and non-antimonial lead automotive batteries are
8 7 =. shown in Table 18.2 and 18.3.
I
*
0 1 2 3 4
Floating time (years) Table 18.2 Typical properties of separators for antimonial
automotive batteries
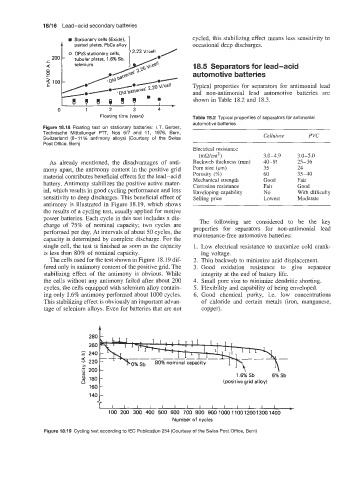
Figure 18.18 Floating test on stationary batteries: I. T. Gerber,
Technische Mitteilunger PlT, Nos 6/7 and 11, 1976, Bern,
Switzerland (8-11 % antimony alloys) (Courtesy of the Swiss Cellulose PVC
Post Office, Bern)
Electrical resistance
(mQ/cm2) 3.0-4.9 3.0-5.0
As already mentioned, the disadvantages of anti- Backweb thickness (mm) 40-91 25-36
mony apart, the antimony content in the positive grid Pore size (pm) 35 24
material contributes beneficial effects for the lead-acid Porosity (%) 60 35-40
battery. Antimony stabilizes the positive active mater- Mechanical strength Good Fair
Good
Fair
Corrosion resistance
ial, which results in good cycling performance and less Enveloping capability No With difficulty
sensitivity to deep discharges. This beneficial effect of Selling price Lowest Moderate
antimony is illustrated in Figure 18.19, which shows
the results of a cycling test, usually applied for motive
power batteries. Each cycle in this test includes a dis-
charge of 75% of nominal capacity; two cycles are The following are considered to be the key
performed per day. At intervals of about 50 cycles, the properties for separators for non-antimonial lead
maintenance-free automotive batteries:
capacity is determined by complete discharge. For the
single cell, the test is finished as soon as the capacity 1. Low electrical resistance to maximize cold crank-
is less than 80% of nominal capacity. ing voltage.
The cells used for the test shown in Figure 18.19 dif- 2. Thin backweb to minimize acid displacement.
fered only in antimony content of the positive grid. The 3. Good oxidation resistance to give separator
stabilizing effect of the antimony is obvious. While integrity at the end of battery life.
the cells without any antimony failed after about 200 4. Small pore size to minimize dendritic shorting.
cycles, the cells equipped with selenium alloy contain- 5. Flexibility and capability of being enveloped.
ing only 1.6% antimony performed about 1000 cycles. 6. Good chemical purity, i.e. low concentrations
This stabilizing effect is obviously an important advan- of chloride and certain metals (iron, manganese,
tage of selenium alloys. Even for batteries that are not copper).
1.6% Sb 6% Sb
u P 180 - (positive grid alloy)
160 -
140 -
L7
I I I I I I I I I I I I I I
Figure 18.19 Cycling test according to IEC Publication 254 (Courtesy of the Swiss Post Office, Bern)