Page 237 - Battery Reference Book
P. 237
Nickel-cadmium secondary batteries 191’3
19.1 Nickel--cadmium secondary necessary, and requires topping up and; in some
batteries cases, a complete electrolyte change. A battery cell
consists of two plates containing nickel hydroxide
Both sealed and open nickel-cadmium batteries are (positive active material) and cadmium hydroxide
based on similar. chemical reactions. The sealed type (negative active material) kept apart by a separ-
is designed to be maintenance free and under nor- ator to maintain electronic insulation. An approxima-
mal conditions will not release gas, whereas an open tion of the chemical equation is given below (see
battery has been designed to release gases when Figure 19.1).
+ - Recombined
-
_t 4
CR CR
T
I 1
I
I I 1.
t- .- .-
4-
1.
t.
0
U
a
P
0
U
.- 1 - -
t.
U 3 a
I
P c c
I
U
B
Y APM 1 1
5
I
DR DR
T T
T
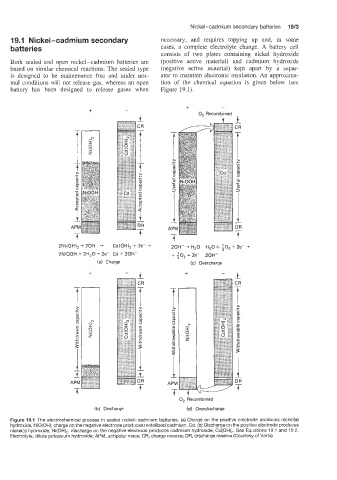
2Ni(OH), + 20H- -+ CdlOH), + 2e- + 20H- + H,O
2NiOOH + 2H,O + 2e- Cd + 20H- t :o, + 2e-
(a) Charge (c) Overcharge
+ L
1
z
z
.- .-
0
0
a %
m
0 a, -
a, -
n n
3
m U
B
E
I DR i
E
f
-0
5
APN T
T
0, Recombined
Ib) Discharge (d) Overdischarge
Figure 19.1 The electrochemical process in sealed nickel-cadmium batteries. (a) Charge on the positive electrode produces nickel(ll1)
hydroxide, NiO(0H); charge on the negative electrode produces metallized cadmium, Cd. (b) Discharge on the positive electrode produces
nickel(i1) hydroxide, Ni(OH),; discharge on the negative electrode produces cadmium hydroxide, Cd(OH),. See Equations 19.1 and 19.2.
Electrolyte, dilute potassium hydroxide; APM, antipolar mass; CR, charge reserve; DR, discharge reserve (Courtesy of Varta)