Page 238 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 238
214 9 Stereoselective Hydrolase-Catalyzed Processes in Continuous-Flow Mode
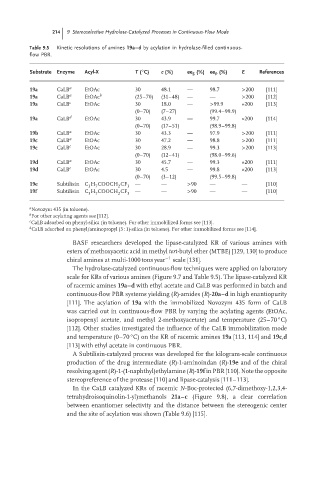
Table 9.5 Kinetic resolutions of amines 19a–d by acylation in hydrolase-filled continuous-
flow PBR.
◦
Substrate Enzyme Acyl-X T ( C) c (%) ee (%) ee (%) E References
S
P
19a CaLB a EtOAc 30 48.1 — 98.7 >200 [111]
19a CaLB a EtOAc b (25–70) (31–48) — — >200 [112]
19a CaLB c EtOAc 30 18.0 — >99.9 »200 [113]
(0–70) (7–27) (99.4–99.9)
19a CaLB d EtOAc 30 43.9 — 99.7 »200 [114]
(0–70) (17–51) (98.9–99.8)
19b CaLB a EtOAc 30 43.3 — 97.9 >200 [111]
19c CaLB a EtOAc 30 47.2 — 98.8 >200 [111]
19c CaLB c EtOAc 30 28.9 — 99.3 >200 [113]
(0–70) (12–41) (98.0–99.6)
19d CaLB a EtOAc 30 45.7 — 99.3 »200 [111]
19d CaLB c EtOAc 30 4.5 — 99.8 »200 [113]
(0–70) (3–12) (99.5–99.8)
19e Subtilisin C H COOCH CF — — >90 — — [110]
3 7 2 3
19f Subtilisin C H COOCH CF 3 — — >90 — — [110]
2
3
7
a Novozym 435 (in toluene).
b
For other acylating agents see [112].
c CaLB adsorbed on phenyl-silica (in toluene). For other immobilized forms see [113].
d
CaLB adsorbed on phenyl/aminopropyl (3 : 1)-silica (in toluene). For other immobilized forms see [114].
BASF researchers developed the lipase-catalyzed KR of various amines with
esters of methoxyacetic acid in methyl tert-butyl ether (MTBE) [129, 130] to produce
chiral amines at multi-1000 tons year −1 scale [131].
The hydrolase-catalyzed continuous-flow techniques were applied on laboratory
scale for KRs of various amines (Figure 9.7 and Table 9.5). The lipase-catalyzed KR
of racemic amines 19a–d with ethyl acetate and CaLB was performed in batch and
continuous-flow PBR systems yielding (R)-amides (R)-20a–d in high enantiopurity
[111]. The acylation of 19a with the immobilized Novozym 435 form of CaLB
was carried out in continuous-flow PBR by varying the acylating agents (EtOAc,
◦
isopropenyl acetate, and methyl 2-methoxyacetate) and temperature (25–70 C)
[112]. Other studies investigated the influence of the CaLB immobilization mode
◦
and temperature (0–70 C) on the KR of racemic amines 19a [113, 114] and 19c,d
[113] with ethyl acetate in continuous PBR.
A Subtilisin-catalyzed process was developed for the kilogram-scale continuous
production of the drug intermediate (R)-1-aminoindan (R)-19e and of the chiral
resolving agent (R)-1-(1-naphthyl)ethylamine (R)-19f in PBR [110]. Note the opposite
stereopreference of the protease [110] and lipase-catalysis [111–113].
In the CaLB catalyzed KRs of racemic N-Boc-protected (6,7-dimethoxy-1,2,3,4-
tetrahydroisoquinolin-1-yl)methanols 21a–c (Figure 9.8), a clear correlation
between enantiomer selectivity and the distance between the stereogenic center
and the site of acylation was shown (Table 9.6) [115].