Page 407 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 407
17.3 Cascade Synthesis of neo-Sialoconjugates 383
the N. meningitidis CSS efficiently catalyzed the formation of fluorinated CMP-sialic
acid derivatives with high yields of the corresponding sialoconjugates 43–45,with
the exception of the equatorially 3F-substituted KDN analog which could not be
activated.
Based on the ability of the E. coli NeuA to produce 9-glycosylated KDN 47 from a
disaccharide 46 aldol acceptor, the synthesis of the unusual α-2,3- and α-2,6-linked
sialylated trisaccharide 48 and tetrasaccharide 49 was reported, in which sialic acids
are linked in both the terminal and reducing positions (Scheme 17.17) [43]. Each
transformation was conducted as a one-pot two-step cascade using a combination
of CSS and one of the complementary SiaT types.
A novel chemoenzymatic method has been developed for the synthesis
of size-defined polysaccharides with sialic acid-containing repeating units by
sialyltransferase-catalyzed block transfer of pseudo-oligosaccharides [70]. The
method is based on the observation that SiaTs with relaxed substrate specificity
can transfer a CMP-activated galactosylated sialic acid to a galactoside acceptor
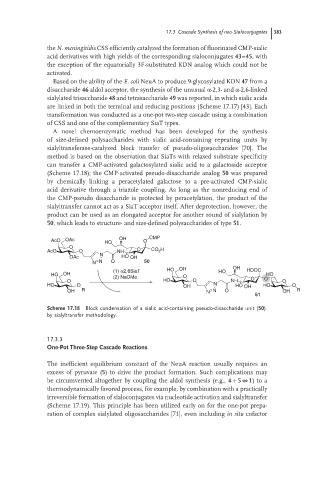
(Scheme 17.18); the CMP-activated pseudo-disaccharide analog 50 was prepared
by chemically linking a peracetylated galactose to a pre-activated CMP-sialic
acid derivative through a triazole coupling. As long as the nonreducing end of
the CMP-pseudo disaccharide is protected by peracetylation, the product of the
sialyltransfer cannot act as a SiaT acceptor itself. After deprotection, however, the
product can be used as an elongated acceptor for another round of sialylation by
50, which leads to structure- and size-defined polysaccharides of type 51.
AcO OAc OH O CMP
HO
O
AcO O NH O CO H
2
OAc N HO OH
N N O 50
(1) α2,6SiaT HO OH HO OH HOOC
HO OH HO
(2) NaOMe O O O
O HO O NH O
HO O OH N HO OH HO O
OH R N N O OH R
51
Scheme 17.18 Block condensation of a sialic acid-containing pseudo-disaccharide unit (50)
by sialyltransfer methodology.
17.3.3
One-Pot Three-Step Cascade Reactions
The inefficient equilibrium constant of the NeuA reaction usually requires an
excess of pyruvate (5) to drive the product formation. Such complications may
be circumvented altogether by coupling the aldol synthesis (e.g., 4 + 5 ⇔ 1)toa
thermodynamically favored process, for example, by combination with a practically
irreversible formation of sialoconjugates via nucleotide activation and sialyltransfer
(Scheme 17.19). This principle has been utilized early on for the one-pot prepa-
ration of complex sialylated oligosaccharides [71], even including in situ cofactor