Page 410 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 410
386 17 Enzymatic Generation of Sialoconjugate Diversity
OH
HO HOOC
O
Y O HOOC HO OH OH
HO OH O O
X O O O O
GD3-type HO OH HO
tetrasaccharide HO OH OH R
57
Pyruvate (7.5 equiv) HO Y
CTP (1.5 equiv) HO O
NeuA, CSS HO OH
HO X CstIIΔ32(I53S)
HO O (1.2 equiv)
HO OH (1.5 equiv)
Pyruvate (5 equiv) HO OH HOOC HO OH OH
CTP (1.5 equiv) O
O O O O
X O
HO OH NeuA, CSS HO OH OH HO R
OH OH
O CstIIΔ32(I53S) 58
HO O O
HO O
OH R OH
OH Pyruvate (5 equiv) Y HOOC
56 CTP (1.5 equiv) HO OH
X O O O
NeuA, CSS HO OH HO O O O
α2,6SiaT OH HO OH R
Y X 59
HO O Pyruvate (7.5 equiv)
CTP (1.5 equiv)
HO OH (1.5 equiv) HO HNAc
CSS
CstII 32(I53S) HO O
OH HO OH
HO HOOC
(1.2 equiv)
O O
AcNH HOOC
HO OH HO OH
X O O O
Y HO OH HO O O O
OH HO OH R
60
α2,8/α2,6-Disialyl lactoside
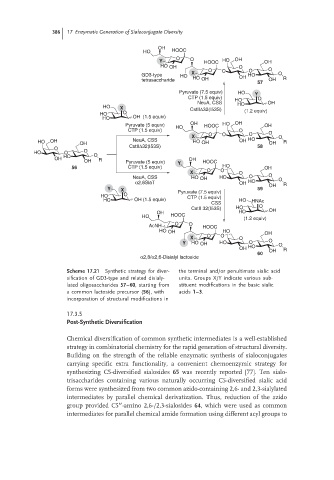
Scheme 17.21 Synthetic strategy for diver- the terminal and/or penultimate sialic acid
sification of GD3-type and related disialy- units. Groups X/Y indicate various sub-
lated oligosaccharides 57–60, starting from stituent modifications in the basic sialic
a common lactoside precursor (56), with acids 1–3.
incorporation of structural modifications in
17.3.5
Post-Synthetic Diversification
Chemical diversification of common synthetic intermediates is a well-established
strategy in combinatorial chemistry for the rapid generation of structural diversity.
Building on the strength of the reliable enzymatic synthesis of sialoconjugates
carrying specific extra functionality, a convenient chemoenzymic strategy for
synthesizing C5-diversified sialosides 65 was recently reported [77]. Ten sialo-
trisaccharides containing various naturally occurring C5-diversified sialic acid
forms were synthesized from two common azido-containing 2,6- and 2,3-sialylated
intermediates by parallel chemical derivatization. Thus, reduction of the azido
′′
group provided C5 -amino 2,6-/2,3-sialosides 64, which were used as common
intermediates for parallel chemical amide formation using different acyl groups to