Page 427 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 427
18.2 SAM-Dependent Methyltransferases 403
outcome of the methylation [39]. Very recently, it was reported that the methyl
donor of the reaction is methylcobalamin [40].
18.2.3
Higher Homologs and Derivatives of SAM
In 1975, Schlenk and Dainko [41] showed that, if yeast was fed with S-n-
propylhomocysteine, an n-propyl analog of SAM could be detected. S-Adenosyl-l-
ethionine was biosynthesized earlier [42].
Synthetic approaches toward SAM analogs that carry other alkyl residues than
methyl have been developed by Dalhoff et al. in 2006 [43]. Other than the simple
alkyl groups (ethyl and n-propyl SAM analogs), the residues carry an allyl or
propargyl group attached directly at the sulfur. On one side, the positively charged
sulfur leads to activation of the transferable methylene group; on the other side,
the same is achieved by the electron-withdrawing effect and cation stabilization by
orbital overlap of the multiple bond. The compounds are thus called double-activated
SAM analogs.
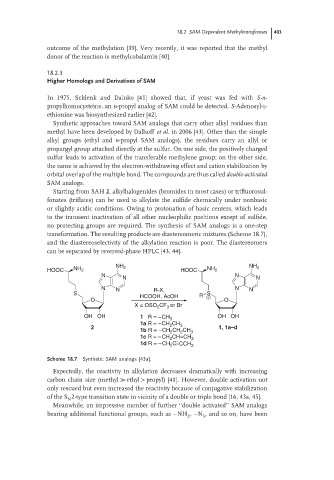
Starting from SAH 2, alkylhalogenides (bromides in most cases) or trifluorosul-
fonates (triflates) can be used to alkylate the sulfide chemically under nonbasic
or slightly acidic conditions. Owing to protonation of basic centers, which leads
to the transient inactivation of all other nucleophilic positions except of sulfide,
no protecting groups are required. The synthesis of SAM analogs is a one-step
transformation. The resulting products are diastereomeric mixtures (Scheme 18.7),
and the diastereoselectivity of the alkylation reaction is poor. The diastereomers
can be separated by reversed-phase HPLC [43, 44].
NH NH
HOOC NH 2 2 HOOC NH 2 2
N N
N N
N N R-X, N N
S HCOOH, AcOH R S
O O
X = OSO 2 CF 3 or Br
OH OH 1 R = –CH 3 OH OH
1a R = –CH CH
2 2 3 1, 1a–d
1b R = –CH CH CH 3
2
2
1c R = –CH 2 CH=CH 2
1d R = –CH C CCH 3
2
Scheme 18.7 Synthetic SAM analogs [43a].
Expectedly, the reactivity in alkylation decreases dramatically with increasing
carbon chain size (methyl ≫ ethyl > propyl) [41]. However, double activation not
only rescued but even increased the reactivity because of conjugative stabilization
of the S 2-type transition state in vicinity of a double or triple bond [16, 43a, 45].
N
Meanwhile, an impressive number of further ‘‘double activated’’ SAM analogs
bearing additional functional groups, such as –NH ,–N , and so on, have been
2 3