Page 432 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 432
408 18 Methyltransferases in Biocatalysis
biologically active form of the cofactor by consumption of inexpensive reagents.
Regeneration systems have been developed for a multitude of cofactors such as
nicotinamide and flavine dinucleotides, coenzyme A esters, or adenosine triphos-
phate (ATP). However, recycling of the methyl donor SAM 1 seems to be a
challenging task [61].
As early as in 1983, Chenault and coworkers [62] proposed that cycling of SAM
might be accomplished by a chemical reaction known from organic synthesis: the
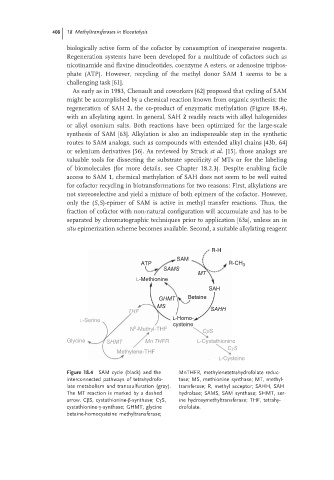
regeneration of SAH 2, the co-product of enzymatic methylation (Figure 18.4),
with an alkylating agent. In general, SAH 2 readily reacts with alkyl halogenides
or alkyl oxonium salts. Both reactions have been optimized for the large-scale
synthesis of SAM [63]. Alkylation is also an indispensable step in the synthetic
routes to SAM analogs, such as compounds with extended alkyl chains [43b, 64]
or selenium derivatives [56]. As reviewed by Struck et al. [15], those analogs are
valuable tools for dissecting the substrate specificity of MTs or for the labeling
of biomolecules (for more details, see Chapter 18.2.3). Despite enabling facile
access to SAM 1, chemical methylation of SAH does not seem to be well suited
for cofactor recycling in biotransformations for two reasons: First, alkylations are
not stereoselective and yield a mixture of both epimers of the cofactor. However,
only the (S,S)-epimer of SAM is active in methyl transfer reactions. Thus, the
fraction of cofactor with non-natural configuration will accumulate and has to be
separated by chromatographic techniques prior to application [63a], unless an in
situ epimerization scheme becomes available. Second, a suitable alkylating reagent
R-H
SAM
ATP R-CH 3
SAMS
MT
L-Methionine
SAH
GHMT Betaine
MS
THF SAHH
L-Homo-
L-Serine
cysteine
5
N -Methyl-THF CβS
Glycine SHMT Mn THFR L-Cystathionine
CγS
Methylene-THF
L-Cysteine
Figure 18.4 SAM cycle (black) and the MnTHFR, methylenetetrahydrofolate reduc-
interconnected pathways of tetrahydrofo- tase; MS, methionine synthase; MT, methyl-
late metabolism and transsulfuration (gray). transferase; R, methyl acceptor; SAHH, SAH
The MT reaction is marked by a dashed hydrolase; SAMS, SAM synthase; SHMT, ser-
arrow. CβS, cystathionine-β-synthase; CγS, ine hydroxymethyltransferase; THF, tetrahy-
cystathionine-γ-synthase; GHMT, glycine drofolate.
betaine-homocysteine methyltransferase;