Page 436 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 436
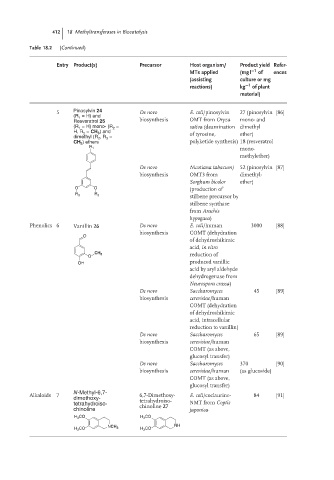
412 18 Methyltransferases in Biocatalysis
Table 18.2 (Continued)
Entry Product(s) Precursor Host organism/ Product yield Refer-
MTs applied (mg l −1 of ences
(assisting culture or mg
reactions) kg −1 of plant
material)
5 Pinosylvin 24 De novo E. coli/pinosylvin 27 (pinosylvin [86]
(R = H) and
1
Resveratrol 25 biosynthesis OMT from Oryza mono- and
(R = H) mono- (R 2 = sativa (deamination dimethyl
1
H, R = CH 3 ) and
2
dimethyl (R 2 , R 3 = of tyrosine, ether)
CH 3 ) ethers polyketide synthesis) 18 (resveratrol
mono-
R 1
methylether)
De novo Nicotiana tabacum/ 52 (pinosylvin [87]
biosynthesis OMT3 from dimethyl-
Sorghum bicolor ether)
O O (production of
stilbene precursor by
R 3 R 3
stilbene synthase
from Arachis
hypogaea)
Phenolics 6 Vanillin 26 De novo E. coli/human 3000 [88]
biosynthesis COMT (dehydration
O
of dehydroshikimic
acid, in vitro
CH 3 reduction of
O
OH produced vanillic
acid by aryl aldehyde
dehydrogenase from
Neurospora crassa)
De novo Saccharomyces 45 [89]
biosynthesis cerevisiae/human
COMT (dehydration
of dehydroshikimic
acid, intracellular
reduction to vanillin)
De novo Saccharomyces 65 [89]
biosynthesis cerevisiae/human
COMT (as above,
glucosyl transfer)
De novo Saccharomyces 370 [90]
biosynthesis cerevisiae/human (as glucoside)
COMT (as above,
glucosyl transfer)
N-Methyl-6,7-
Alkaloids 7 6,7-Dimethoxy- E. coli/coclaurine- 84 [91]
dimethoxy- tetrahydroiso-
tetrahydroiso- chinoline 27 NMT from Coptis
chinoline japonica
H 3 CO H 3 CO
NH
H 3 CO NCH 3 H 3 CO