Page 437 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 437
18.2 SAM-Dependent Methyltransferases 413
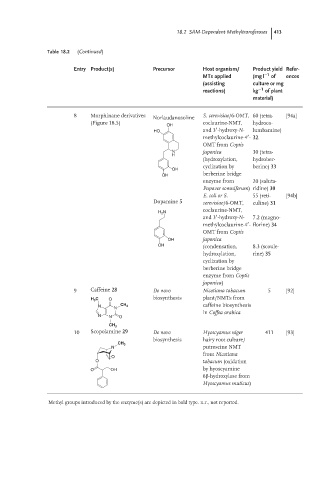
Table 18.2 (Continued)
Entry Product(s) Precursor Host organism/ Product yield Refer-
MTs applied (mg l −1 of ences
(assisting culture or mg
reactions) kg −1 of plant
material)
8 Morphinane derivatives Norlaudanosoline S. cerevisiae/6-OMT, 60 (tetra- [94a]
(Figure 18.5) coclaurine-NMT, hydroco-
OH
′
HO and 3 -hydroxy-N- lumbamine)
′
methylcoclaurine-4 - 32
OMT from Coptis
N japonica 30 (tetra-
H
(hydroxylation, hydrober-
cyclization by berine) 33
OH
OH berberine bridge
enzyme from 20 (saluta-
Papaver somniferum) ridine) 30
E. coli or S. 55 (reti- [94b]
Dopamine 5 cerevisiae/6-OMT, culine) 31
coclaurine-NMT,
H 2 N
′
and 3 -hydroxy-N- 7.2 (magno-
′
methylcoclaurine-4 - florine) 34
OMT from Coptis
OH japonica
OH (condensation, 8.3 (scoule-
hydroxylation, rine) 35
cyclization by
berberine bridge
enzyme from Coptis
japonica)
9 Caffeine 28 De novo Nicotiana tabacum 5 [92]
H 3 C O biosynthesis plant/NMTs from
N CH 3 caffeine biosynthesis
N
in Coffea arabica
N N O
CH 3
10 Scopolamine 29 De novo Hyoscyamus niger 411 [93]
biosynthesis hairy root culture/
CH 3
N putrescine NMT
from Nicotiana
O
O tabacum (oxidation
O OH by hyoscyamine
6β-hydroxylase from
Hyoscyamus muticus)
Methyl groups introduced by the enzyme(s) are depicted in bold type. n.r., not reported.