Page 70 - Biodegradable Polyesters
P. 70
48 3 Microbial Synthesis of Biodegradable Polyesters: Processes, Products, Applications
(R)-3-hydroxybutyryl-CoA monomers are generated from acetyl-CoA by the
action of two other enzymes [11, 12]. The three PHB biosynthesis genes are
ordered in one operon, the phaCAB operon.
β-Ketothiolase (encoded by phaA) condenses two molecules of acetyl-CoA
to acetoacetyl-CoA, which is then reduced to (R)-3-hydroxybutyryl-CoA by
the NADPH-dependent acetoacetyl-CoA reductase (encoded by phaB). The
PHB synthase (encoded by phaC in R. eutropha R.) then converts the thioester
monomers into the polyoxoester PHB. The polymer aggregates to form a spher-
ical inclusion or granule usually 50–500 nm in diameter with the amorphous
hydrophobic PHA polyester at the core and attached or embedded proteins at
the surface, including the PHA synthase, PHA depolymerases, structural, and
regulatory proteins [13, 14].
This chapter discusses the present literature on bacterial PHA synthesis, PHA
granules, their biogenesis, and structure, and on protein engineering approaches
of associated proteins aiming at the design of PHA granules as biobeads for
biomedical use.
3.2
Biogenesis of Microbial Polyhydroxyalkanoate Granules
Microorganisms can form a range of intracellular and spherical inclusions. The
inclusions can be surrounded by a phospholipid membrane and separated into
inorganic inclusions, such as magnetosomes (iron oxide core) and organic inclu-
sions such as biopolyester (PHAs) granules (polyester core). The polyester syn-
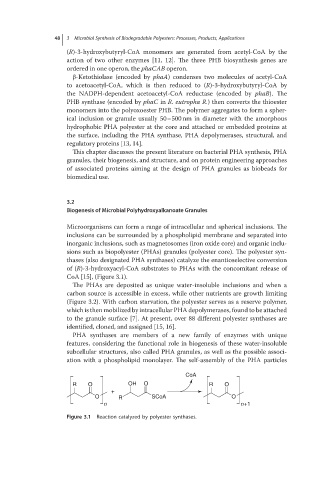
thases (also designated PHA synthases) catalyze the enantioselective conversion
of (R)-3-hydroxyacyl-CoA substrates to PHAs with the concomitant release of
CoA [15], (Figure 3.1).
The PHAs are deposited as unique water-insoluble inclusions and when a
carbon source is accessible in excess, while other nutrients are growth limiting
(Figure 3.2). With carbon starvation, the polyester serves as a reserve polymer,
which is then mobilized by intracellular PHA depolymerases, found to be attached
to the granule surface [7]. At present, over 88 different polyester synthases are
identified, cloned, and assigned [15, 16].
PHA synthases are members of a new family of enzymes with unique
features, considering the functional role in biogenesis of these water-insoluble
subcellular structures, also called PHA granules, as well as the possible associ-
ation with a phospholipid monolayer. The self-assembly of the PHA particles
CoA
R O OH O R O
+
O R SCoA O
n n+1
Figure 3.1 Reaction catalyzed by polyester synthases.