Page 75 - Biodegradable Polyesters
P. 75
3.6 PHA Inclusions: Self-Assembly and Structure 53
Polyester O
synthase Polyester Polyester
synthase synthase
SH CoA S OH
S
O
SH
Substrate O O
binding CoA Polymerization O O
HO
Polyester Dimerization Polyester
synthase Polyester synthase
S synthase
O S S
O O
OH
OH HO
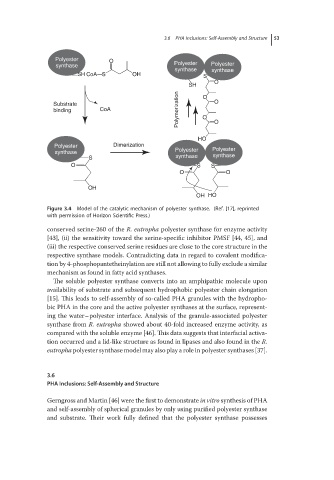
Figure 3.4 Model of the catalytic mechanism of polyester synthase. (Ref. [17], reprinted
with permission of Horizon Scientific Press.)
conserved serine-260 of the R. eutropha polyester synthase for enzyme activity
[43], (ii) the sensitivity toward the serine-specific inhibitor PMSF [44, 45], and
(iii) the respective conserved serine residues are close to the core structure in the
respective synthase models. Contradicting data in regard to covalent modifica-
tion by 4-phosphopantetheinylation are still not allowing to fully exclude a similar
mechanism as found in fatty acid synthases.
The soluble polyester synthase converts into an amphipathic molecule upon
availability of substrate and subsequent hydrophobic polyester chain elongation
[15]. This leads to self-assembly of so-called PHA granules with the hydropho-
bic PHA in the core and the active polyester synthases at the surface, represent-
ing the water–polyester interface. Analysis of the granule-associated polyester
synthase from R. eutropha showed about 40-fold increased enzyme activity, as
compared with the soluble enzyme [46]. This data suggests that interfacial activa-
tion occurred and a lid-like structure as found in lipases and also found in the R.
eutropha polyester synthase model may also play a role in polyester synthases [37].
3.6
PHA Inclusions: Self-Assembly and Structure
Gerngross and Martin [46] were the first to demonstrate in vitro synthesis of PHA
and self-assembly of spherical granules by only using purified polyester synthase
and substrate. Their work fully defined that the polyester synthase possesses