Page 73 - Biodegradable Polyesters
P. 73
3.4 Polyester (PHA) Synthases are the Key Enzymes 51
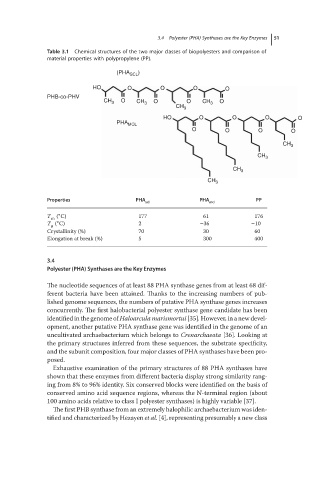
Table 3.1 Chemical structures of the two major classes of biopolyesters and comparison of
material properties with polypropylene (PP).
(PHA SCL )
HO O O O O
PHB-co-PHV
CH 3 O CH 3 O O CH 3 O
CH 3
HO O O O O
PHA MCL
O O O O
CH 3
CH 3
CH 3
CH 3
Properties PHA PHA PP
scl mcl
∘
T m ( C) 177 61 176
∘
T ( C) 2 −36 −10
g
Crystallinity (%) 70 30 60
Elongation at break (%) 5 300 400
3.4
Polyester (PHA) Synthases are the Key Enzymes
The nucleotide sequences of at least 88 PHA synthase genes from at least 68 dif-
ferent bacteria have been attained. Thanks to the increasing numbers of pub-
lished genome sequences, the numbers of putative PHA synthase genes increases
concurrently. The first halobacterial polyester synthase gene candidate has been
identified in the genome of Haloarcula marismortui [35]. However, in a new devel-
opment, another putative PHA synthase gene was identified in the genome of an
uncultivated archaebacterium which belongs to Crenarchaeota [36]. Looking at
the primary structures inferred from these sequences, the substrate specificity,
and the subunit composition, four major classes of PHA synthases have been pro-
posed.
Exhaustive examination of the primary structures of 88 PHA synthases have
shown that these enzymes from different bacteria display strong similarity rang-
ing from 8% to 96% identity. Six conserved blocks were identified on the basis of
conserved amino acid sequence regions, whereas the N-terminal region (about
100 amino acids relative to class I polyester synthases) is highly variable [37].
The first PHB synthase from an extremely halophilic archaebacterium was iden-
tified and characterized by Hezayen et al. [4], representing presumably a new class