Page 227 - Biofuels Refining and Performance
P. 227
210 Chapter Seven
Methane
Methanol
Iron
Oxygen
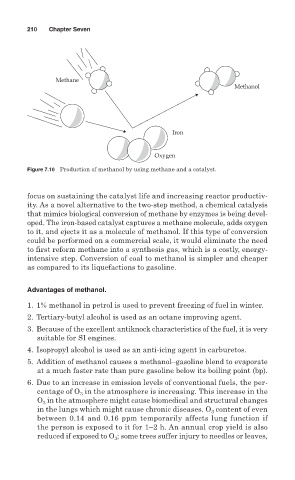
Figure 7.10 Production of methanol by using methane and a catalyst.
focus on sustaining the catalyst life and increasing reactor productiv-
ity. As a novel alternative to the two-step method, a chemical catalysis
that mimics biological conversion of methane by enzymes is being devel-
oped. The iron-based catalyst captures a methane molecule, adds oxygen
to it, and ejects it as a molecule of methanol. If this type of conversion
could be performed on a commercial scale, it would eliminate the need
to first reform methane into a synthesis gas, which is a costly, energy-
intensive step. Conversion of coal to methanol is simpler and cheaper
as compared to its liquefactions to gasoline.
Advantages of methanol.
1. 1% methanol in petrol is used to prevent freezing of fuel in winter.
2. Tertiary-butyl alcohol is used as an octane improving agent.
3. Because of the excellent antiknock characteristics of the fuel, it is very
suitable for SI engines.
4. Isopropyl alcohol is used as an anti-icing agent in carburetos.
5. Addition of methanol causes a methanol–gasoline blend to evaporate
at a much faster rate than pure gasoline below its boiling point (bp).
6. Due to an increase in emission levels of conventional fuels, the per-
in the atmosphere is increasing. This increase in the
centage of O 3
O in the atmosphere might cause biomedical and structural changes
3
in the lungs which might cause chronic diseases. O content of even
3
between 0.14 and 0.16 ppm temporarily affects lung function if
the person is exposed to it for 1–2 h. An annual crop yield is also
reduced if exposed to O ; some trees suffer injury to needles or leaves,
3