Page 228 - Biofuels Refining and Performance
P. 228
Ethanol and Methanol as Fuels in Internal Combustion Engines 211
0.8
0.7 0.637 5.3% reduction
Grams ozone per mile 0.5 0.301 0.396 40% reduction
0.6
0.4
0.3
0.236
0.2
0.1
0
GTMV FFV
Gasoline Methanol
(Indolene) (M85)
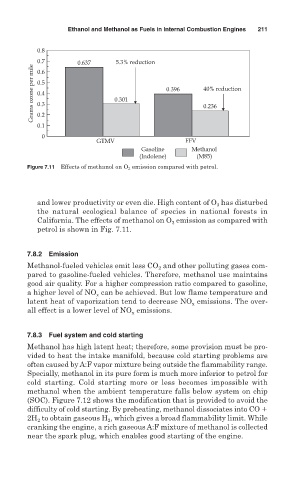
Figure 7.11 Effects of methanol on O 3 emission compared with petrol.
and lower productivity or even die. High content of O 3 has disturbed
the natural ecological balance of species in national forests in
California. The effects of methanol on O 3 emission as compared with
petrol is shown in Fig. 7.11.
7.8.2 Emission
Methanol-fueled vehicles emit less CO 2 and other polluting gases com-
pared to gasoline-fueled vehicles. Therefore, methanol use maintains
good air quality. For a higher compression ratio compared to gasoline,
can be achieved. But low flame temperature and
a higher level of NO x
latent heat of vaporization tend to decrease NO emissions. The over-
x
all effect is a lower level of NO emissions.
x
7.8.3 Fuel system and cold starting
Methanol has high latent heat; therefore, some provision must be pro-
vided to heat the intake manifold, because cold starting problems are
often caused by A:F vapor mixture being outside the flammability range.
Specially, methanol in its pure form is much more inferior to petrol for
cold starting. Cold starting more or less becomes impossible with
methanol when the ambient temperature falls below system on chip
(SOC). Figure 7.12 shows the modification that is provided to avoid the
difficulty of cold starting. By preheating, methanol dissociates into CO
2H to obtain gaseous H , which gives a broad flammability limit. While
2
2
cranking the engine, a rich gaseous A:F mixture of methanol is collected
near the spark plug, which enables good starting of the engine.