Page 91 - Biomass Gasification, Pyrolysis And Torrefaction Practical Design and Theory
P. 91
Chapter | 3 Biomass Characteristics 69
3.5.2.2 Specific Heat
Specific heat is an important thermodynamic property of biomass often required
for thermodynamic calculations. It is an indication of the heat capacity of a sub-
stance. Both moisture and temperature affect the specific heat of biomass, but
density or wood species do not have much effect on the specific heat (Ragland
et al., 1991). The specific heat changes muchwithtemperature.Italsodepends to
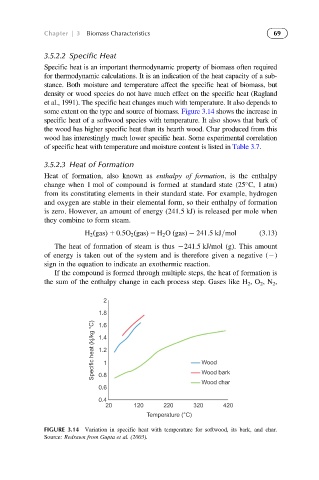
some extent on the type and source of biomass. Figure 3.14 shows the increase in
specific heat of a softwood species with temperature. It also shows that bark of
the wood has higher specific heat than its hearth wood. Char produced from this
wood has interestingly much lower specific heat. Some experimental correlation
of specific heat with temperature and moisture content is listed in Table 3.7.
3.5.2.3 Heat of Formation
Heat of formation, also known as enthalpy of formation, is the enthalpy
change when 1 mol of compound is formed at standard state (25 C, 1 atm)
from its constituting elements in their standard state. For example, hydrogen
and oxygen are stable in their elemental form, so their enthalpy of formation
is zero. However, an amount of energy (241.5 kJ) is released per mole when
they combine to form steam.
H 2 ðgasÞ 1 0:5O 2 ðgasÞ 5 H 2 O ðgasÞ 241:5kJ=mol (3.13)
The heat of formation of steam is thus 2241.5 kJ/mol (g). This amount
of energy is taken out of the system and is therefore given a negative (2)
sign in the equation to indicate an exothermic reaction.
If the compound is formed through multiple steps, the heat of formation is
the sum of the enthalpy change in each process step. Gases like H 2 ,O 2 ,N 2 ,
2
1.8
Specific heat (kj/kg °C) 1.4 1 Wood
1.6
1.2
Wood bark
0.8
Wood char
0.6
0.4
20 120 220 320 420
Temperature (°C)
FIGURE 3.14 Variation in specific heat with temperature for softwood, its bark, and char.
Source: Redrawn from Gupta et al. (2003).