Page 378 - Biomedical Engineering and Design Handbook Volume 2, Applications
P. 378
356 DIAGNOSTIC EQUIPMENT DESIGN
12.4 NEW AND DEVELOPING BREAST IMAGING MODALITIES
12.4.1 Introduction
While x-ray mammography is unquestionably the leading currently available modality for early detec-
tion of small cancers, it suffers from a relatively low positive predictive value (the fraction of lesions
identified as positive that ultimately turn out to be positive). As a result, 65 to 85 percent of all breast
biopsies are negative (Kerlikowske et al., 1993; Kopans, 1992). Therefore, adjunct modalities that can
differentiate benign and malignant lesions detected by mammography are considered highly desirable.
In a report issued by the National Research Council in 2000, the Committee on Technologies for the
Early Detection of Breast Cancer identified the breast imaging technologies and their current status
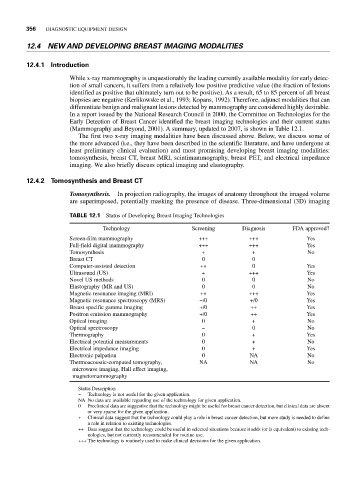
(Mammography and Beyond, 2001). A summary, updated to 2007, is shown in Table 12.1.
The first two x-ray imaging modalities have been discussed above. Below, we discuss some of
the more advanced (i.e., they have been described in the scientific literature, and have undergone at
least preliminary clinical evaluation) and most promising developing breast imaging modalities:
tomosynthesis, breast CT, breast MRI, scintimammography, breast PET, and electrical impedance
imaging. We also briefly discuss optical imaging and elastography.
12.4.2 Tomosynthesis and Breast CT
Tomosynthesis. In projection radiography, the images of anatomy throughout the imaged volume
are superimposed, potentially masking the presence of disease. Three-dimensional (3D) imaging
TABLE 12.1 Status of Developing Breast Imaging Technologies
Technology Screening Diagnosis FDA approved?
Screen-film mammography +++ +++ Yes
Full-field digital mammography +++ +++ Yes
Tomosynthesis + + No
Breast CT 0 0
Computer-assisted detection ++ 0 Yes
Ultrasound (US) + +++ Yes
Novel US methods 0 0 No
Elastography (MR and US) 0 0 No
Magnetic resonance imaging (MRI) ++ +++ Yes
Magnetic resonance spectroscopy (MRS) −/0 +/0 Yes
Breast specific gamma imaging +/0 ++ Yes
Positron emission mammography +/0 ++ Yes
Optical imaging 0 + No
Optical spectroscopy − 0 No
Thermography 0 + Yes
Electrical potential measurements 0 + No
Electrical impedance imaging 0 + Yes
Electronic palpation 0 NA No
Thermoacoustic-computed tomography, NA NA No
microwave imaging, Hall effect imaging,
magnetomammography
Status Description
− Technology is not useful for the given application.
NA No data are available regarding use of the technology for given application.
0 Preclinical data are suggestive that the technology might be useful for breast cancer detection, but clinical data are absent
or very sparse for the given application.
+ Clinical data suggest that the technology could play a role in breast cancer detection, but more study is needed to define
a role in relation to existing technologies.
++ Data suggest that the technology could be useful in selected situations because it adds (or is equivalent) to existing tech-
nologies, but not currently recommended for routine use.
+++ The technology is routinely used to make clinical decisions for the given application.