Page 165 - Carbon Nanotubes
P. 165
156 Y. SAITO
electron per formula unit, RC,)[22] (i.e., metallic
electrical properties) though they are carbides. All the
lanthanide carbides including YC, and Sc3C, are hy-
groscopic; they quickly react with water in air and 10'
hydrolyze, emanating hydrogen and acetylene. There-
fore, they usually have to be treated and stored in an
inactive gas atmosphere or oil to avoid hydrolysis.
However, the observation of intact dicarbides, even 100
after exposure to air for over a year, shows the excel-
Y
lent airtight nature of nanocapsules, and supports i2
the hypothesis that their structure is completely closed v1
a,
by introducing pentagons into graphitic sheets like v1
& 10-l
fullerenes[23]. 5
4.1.2 Correlation between metal volatility and F
encapsulation. A glance at Table 1 shows us that
carbon nanocapsules stuffed with metal carbides are
formed for most of the rare-earth metals, Sc, Y, La,
Ce, Pr, Nd, Gd, Tb, Dy, Ho, Er, and Lu. Both TEM
and XRD confirm the formation of encapsulated car-
bides for all the above elements. The structural and
morphological features described above for Y are 10
common to all the stuffed nanocapsules: the outer 1000 1500 2000 2500 3000
shell, being made up of concentric multilayered gra-
phitic sheets, is polyhedral, and the inner space is par- Temperature [K]
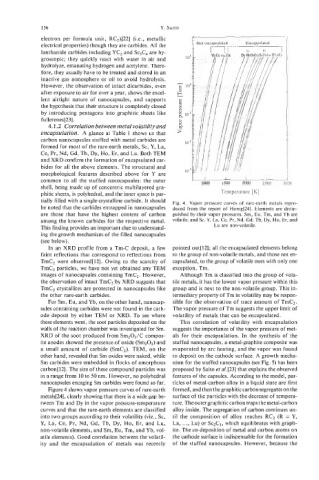
tially filled with a single-crystalline carbide. It should Fig. 4. Vapor pressure curves of rare-earth metals repro-
be noted that the carbides entrapped in nanocapsules duced from the report of Honig[24]. Elements are distin-
are those that have the highest content of carbon guished by their vapor pressures. Sm, ELI, Tm, and Yb are
among the known carbides for the respective metal. volatile, and Sc, Y, La, Ce, Pr, Nd, Gd, Tb, Dy, Ho, Er, and
This finding provides an important clue to understand- Lu are non-volatile.
ing the growth mechanism of the filled nanocapsules
(see below).
In an XRD profile from a Tm-C deposit, a few pointed out[ 121; all the encapsulated elements belong
faint reflections that correspond to reflections from to the group of non-volatile metals, and those not en-
TmC, were observed[l2]. Owing to the scarcity of capsulated, to the group of volatile ones with only one
TmC, particles, we have not yet obtained any TEM exception, Tm.
images of nanocapsules containing TmC,. However, Although Tm is classified into the group of vola-
the observation of intact TmC, by XRD suggests that tile metals, it has the lowest vapor pressure within this
TmC, crystallites are protected in nanocapsules like group and is next to the non-volatile group. This in-
the other rare-earth carbides. termediary property of Tm in volatility may be respon-
For Sm, Eu, and Yb, on the other hand, nanocap- sible for the observation of trace amount of TmC2.
sules containing carbides were not found in the cath- The vapor pressure of Tm suggests the upper limit of
ode deposit by either TEM or XRD. To see where volatility of metals that can be encapsulated.
these elements went, the soot particles deposited on the This correlation of volatility with encapsulation
walls of the reaction chamber was investigated for Sm. suggests the importance of the vapor pressure of met-
XRD of the soot produced from Sm203/C compos- als for their encapsulation. In the synthesis of the
ite anodes showed the presence of oxide (Sm203) and stuffed nanocapsules, a metal-graphite composite was
a small amount of carbide (SmC,). TEM, on the evaporated by arc heating, and the vapor was found
other hand, revealed that Sm oxides were naked, while to deposit on the cathode surface. A growth mecha-
Sm carbides were embedded in flocks of amorphous nism for the stuffed nanocapsules (see Fig. 5) has been
carbon[l2]. The size of these compound particles was proposed by Saito et a1.[23] that explains the observed
in a range from 10 to 50 nm. However, no polyhedral features of the capsules. According to the model, par-
nanocapsules encaging Sm carbides were found so far. ticles of metal-carbon alloy in a liquid state are first
Figure 4 shows vapor pressure curves of rare-earth formed, and then the graphitic carbon segregates on the
metals[24], clearly showing that there is a wide gap be- surface of the particles with the decrease of tempera-
tween Tm and Dy in the vapor pressure-temperature ture. The outer graphitic carbon traps the metal-carbon
curves and that the rare-earth elements are classified alloy inside. The segregation of carbon continues un-
into two groups according to their volatility (viz., Sc, til the composition of alloy reaches RC2 (R = Y,
Y, La, Ce, Pr, Nd, Gd, Tb, Dy, Ho, Er, and Lu, La, . . . , Lu) or Sc2C3, which equilibrates with graph-
non-volatile elements, and Sm, Eu, Tm, and Yb, vol- ite. The co-deposition of metal and carbon atoms on
atile elements). Good correlation between the volatil- the cathode surface is indispensable for the formation
ity and the encapsulation of metals was recently of the stuffed nanocapsules. However, because the