Page 170 - Carbon Nanotubes
P. 170
Nanoparticles and filled nanocapsules 161
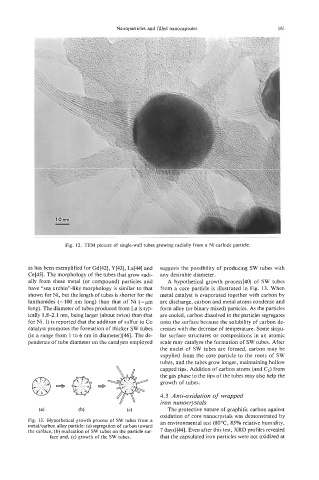
Fig. 12. TEM picture of single-wall tubes growing radially from a Ni-carbide particle.
as has been exemplified for Gd[42], Y[43], La[44] and suggests the possibility of producing SW tubes with
Ce[45]. The morphology of the tubes that grow radi- any desirable diameter.
ally from these metal (or compound) particles and A hypothetical growth process[40] of SW tubes
have “sea urchin”-like morphology is similar to that from a core particle is illustrated in Fig. 13. When
shown for Ni, but the length of tubes is shorter for the metal catalyst is evaporated together with carbon by
lanthanides (-100 nm long) than that of Ni (-pm arc discharge, carbon and metal atoms condense and
long). The diameter of tubes produced from La is typ- form alloy (or binary mixed) particles. As the particles
ically 1.8-2.1 nm, being larger (about twice) than that are cooled, carbon dissolved in the particles segregates
for Ni. It is reported that the addition of sulfur to Co onto the surface because the solubility of carbon de-
catalyst promotes the formation of thicker SW tubes creases with the decrease of temperature. Some singu-
(in a range from 1 to 6 nm in diameter)[46]. The de- lar surface structures or compositions in an atomic
pendence of tube diameter on the catalysts employed scale may catalyze the formation of SW tubes. After
the nuclei of SW tubes are formed, carbon may be
supplied from the core particle to the roots of SW
tubes, and the tubes grow longer, maintaining hollow
capped tips. Addition of carbon atoms (and C,) from
the gas phase to the tips of the tubes may also help the
growth of tubes.
4.3 Anti-oxidation of wrapped
iron nanocrystals
(a) (b) (C) The protective nature of graphitic carbon against
oxidation of core nanocrystals was demonstrated by
Fig. 13. Hypothetical growth process of SW tubes from a an environmental test (80°C, 85% relative humidity,
metal/carbon alloy particle: (a) segregation of carbon toward
the surface, (b) nucleation of SW tubes on the particle sur- 7 days)[44]. Even after this test, XRD profiles revealed
face and, (c) growth of the SW tubes. that the capsulated iron particles were not oxidized at