Page 166 - Carbon Nanotubes
P. 166
Nanoparticles and filled nanocapsules 157
(a) (b) (C)
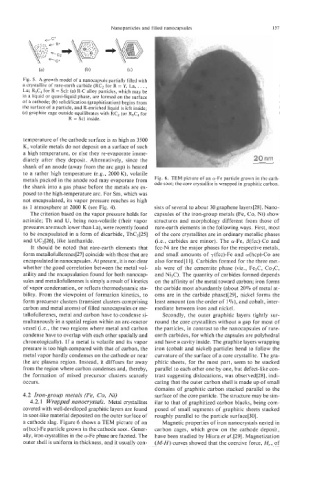
Fig. 5. A growth model of a nanocapsule partially filled with
a crystallite of rare-earth carbide (RC, for R = Y, La, . . . ,
Lu; R,C, for R = Sc): (a) R-C alloy particles, which may be
in a liquid or quasi-liquid phase, are formed on the surface
of a cathode; (b) solidification (graphitization) begins from
the surface of a particle, and R-enriched liquid is left inside;
(c) graphite cage outside equilibrates with RC, (or R3C4 for
R = Sc) inside.
temperature of the cathode surface is as high as 3500
K, volatile metals do not deposit on a surface of such
a high temperature, or else they re-evaporate imme-
diately after they deposit. Alternatively, since the
shank of an anode (away from the arc gap) is heated
to a rather high temperature (e.g., 2000 K), volatile
metals packed in the anode rod may evaporate from Fig. 6. TEM picture of an a-Fe particle grown in the cath-
ode soot; the core crystallite is wrapped in graphitic carbon.
the shank into a gas phase before the metals are ex-
posed to the high-temperature arc. For Sm, which was
not encapsulated, its vapor pressure reaches as high
as 1 atmosphere at 2000 K (see Fig. 4). sists of several to about 30 graphene layers[28]. Nano-
The criterion based on the vapor pressure holds for capsules of the iron-group metals (Fe, Co, Ni) show
actinide; Th and U, being non-volatile (their vapor structures and morphology different from those of
pressures are much lower than La), were recently found rare-earth elements in the following ways. First, most
to be encapsulated in a form of dicarbide, ThC2[25] of the core crystallites are in ordinary metallic phases
and UC2[26], like lanthanide. (Le., carbides are minor). The a-Fe, P(fcc)-Co and
It should be noted that rare-earth elements that fcc-Ni are the major phases for the respective metals,
form metallofullerenes[27] coincide with those that are and small amounts of y(fcc)-Fe and a(hcp)-Co are
encapsulated in nanocapsules. At present, it is not clear also formed[ll]. Carbides formed for the three met-
whether the good correlation between the metal vol- als were of the cementite phase (viz., Fe3C, Co3C,
atility and the encapsulation found for both nanocap- and Ni3C). The quantity of carbides formed depends
sules and metallofullerenes is simply a result of kinetics on the affinity of the metal toward carbon; iron forms
of vapor condensation, or reflects thermodynamic sta- the carbide most abundantly (about 20% of metal at-
bility. From the viewpoint of formation kinetics, to oms are in the carbide phase)[29], nickel forms the
form precursor clusters (transient clusters comprising least amount (on the order of lOro), and cobalt, inter-
carbon and metal atoms) of filled nanocapsules or me- mediate between iron and nickel.
tallofullerenes, metal and carbon have to condense si- Secondly, the outer graphitic layers tightly sur-
multaneously in a spatial region within an arc-reactor round the core crystallites without a gap for most of
vessel (i.e., the two regions where metal and carbon the particles, in contrast to the nanocapsules of rare-
condense have to overlap with each other spatially and earth carbides, for which the capsules are polyhedral
chronologically). If a metal is volatile and its vapor and have a cavity inside. The graphite layers wrapping
pressure is too high compared with that of carbon, the iron (cobalt and nickel) particles bend to follow the
metal vapor hardly condenses on the cathode or near curvature of the surface of a core crystallite. The gra-
the arc plasma region. Instead, it diffuses far away phitic sheets, for the most part, seem to be stacked
from the region where carbon condenses and, thereby, parallel to each other one by one, but defect-like con-
the formation of mixed precursor clusters scarcely trast suggesting dislocations, was observed[28], indi-
occurs. cating that the outer carbon shell is made up of small
domains of graphitic carbon stacked parallel to the
4.2 Iron-group metals (Fe, Co, Ni) surface of the core particle. The structure may be sim-
4.2.1 Wrapped nanocrystals. Metal crystallites ilar to that of graphitized carbon blacks, being com-
covered with well-developed graphitic layers are found posed of small segments of graphitic sheets stacked
in soot-like material deposited on the outer surface of roughly parallel to the particle surface[30].
a cathode slag. Figure 6 shows a TEM picture of an Magnetic properties of iron nanocrystals nested in
a(bcc)-Fe particle grown in the cathode soot. Gener- carbon cages, which grew on the cathode deposit,
ally, iron crystallites in the a-Fe phase are faceted. The have been studied by Hiura et al. [29]. Magnetization
outer shell is uniform in thickness, and it usually con- (M-H) curves showed that the coercive force, H,, of