Page 169 - Carbon Nanotubes
P. 169
160 Y. SAITO
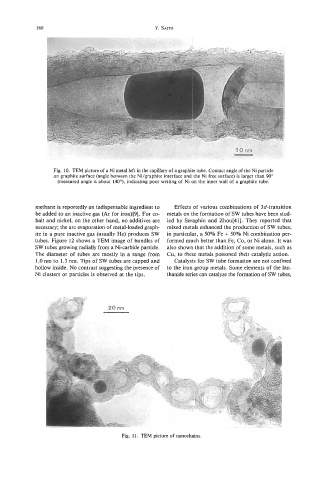
Fig. 10. TEM picture of a Ni metal left in the capillary of a graphite tube. Contact angle of the Ni particle
on graphite surface (angle between the Ni/graphite interface and the Ni free surface) is larger than 90"
(measured angle is about 140"), indicating poor wetting of Ni on the inner wall of a graphite tube.
methane is reportedly an indispensable ingredient to Effects of various combinations of 3d-transition
be added to an inactive gas (Ar for iron)[9]. For co- metals on the formation of SW tubes have been stud-
balt and nickel, on the other hand, no additives are ied by Seraphin and Zhou[41]. They reported that
necessary; the arc evaporation of metal-loaded graph- mixed metals enhanced the production of SW tubes;
ite in a pure inactive gas (usually He) produces SW in particular, a 50% Fe + 50% Ni combination per-
tubes. Figure 12 shows a TEM image of bundles of formed much better than Fe, Co, or Ni alone. It was
SW tubes growing radially from a Ni-carbide particle. also shown that the addition of some metals, such as
The diameter of tubes are mostly in a range from Cu, to these metals poisoned their catalytic action.
1.0 nm to 1.3 nm. Tips of SW tubes are capped and Catalysts for SW tube formation are not confined
hollow inside. No contrast suggesting the presence of to the iron-group metals. Some elements of the lan-
Ni clusters or particles is observed at the tips. thanide series can catalyze the formation of SW tubes,
Fig. 11. TEM picture of nanochains.