Page 159 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 159
122 Carraher’s Polymer Chemistry
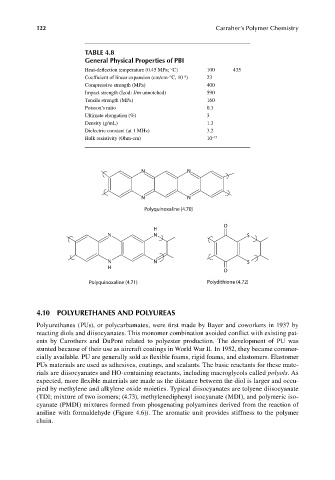
TABLE 4.8
General Physical Properties of PBI
Heat-deflection temperature (0.45 MPa; °C) 100 435
–6
Coefficient of linear expansion (cm/cm-°C, 10 ) 23
Compressive strength (MPa) 400
Impact strength (Izod: J/m unnotched) 590
Tensile strength (MPa) 160
Poisson’s ratio 0.3
Ultimate elongation (%) 3
Density (g/mL) 1.3
Dielectric constant (at 1 MHz) 3.2
Bulk resistivity (Ohm-cm) 10 –15
N N
N N
Polyquinoxaline (4.70)
O
H
N N S
N N S
H
O
Polyquinoxaline (4.71) Polydithione (4.72)
4.10 POLYURETHANES AND POLYUREAS
Polyurethanes (PUs), or polycarbamates, were first made by Bayer and coworkers in 1937 by
reacting diols and diisocyanates. This monomer combination avoided conflict with existing pat-
ents by Carothers and DuPont related to polyester production. The development of PU was
stunted because of their use as aircraft coatings in World War II. In 1952, they became commer-
cially available. PU are generally sold as flexible foams, rigid foams, and elastomers. Elastomer
PUs materials are used as adhesives, coatings, and sealants. The basic reactants for these mate-
rials are diisocyanates and HO-containing reactants, including macroglycols called polyols. As
expected, more fl exible materials are made as the distance between the diol is larger and occu-
pied by methylene and alkylene oxide moieties. Typical diisocyanates are tolyene diisocyanate
(TDI; mixture of two isomers; (4.73), methylenediphenyl isocyanate (MDI), and polymeric iso-
cyanate (PMDI) mixtures formed from phosgenating polyamines derived from the reaction of
aniline with formaldehyde (Figure 4.6)). The aromatic unit provides stiffness to the polymer
chain.
9/14/2010 3:38:25 PM
K10478.indb 122
K10478.indb 122 9/14/2010 3:38:25 PM