Page 163 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 163
126 Carraher’s Polymer Chemistry
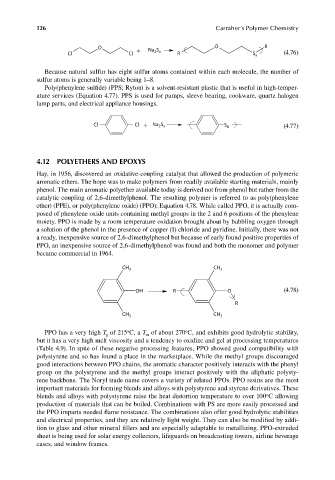
O + Na S O R
2 x
Cl Cl R S x (4.76)
Because natural sulfur has eight sulfur atoms contained within each molecule, the number of
sulfur atoms is generally variable being 1–8.
Poly(phenylene sulfide) (PPS; Ryton) is a solvent-resistant plastic that is useful in high-temper-
ature services (Equation 4.77). PPS is used for pumps, sleeve bearing, cookware, quartz halogen
lamp parts, and electrical appliance housings.
Cl Cl + Na S S x (4.77)
2 x
4.12 POLYETHERS AND EPOXYS
Hay, in 1956, discovered an oxidative-coupling catalyst that allowed the production of polymeric
aromatic ethers. The hope was to make polymers from readily available starting materials, mainly
phenol. The main aromatic polyether available today is derived not from phenol but rather from the
catalytic coupling of 2,6-dimethylphenol. The resulting polymer is referred to as poly(phenylene
ether) (PPE), or poly(phenylene oxide) (PPO); Equation 4.78. While called PPO, it is actually com-
posed of phenylene oxide units containing methyl groups in the 2 and 6 positions of the phenylene
moiety. PPO is made by a room temperature oxidation brought about by bubbling oxygen through
a solution of the phenol in the presence of copper (I) chloride and pyridine. Initially, there was not
a ready, inexpensive source of 2,6-dimethylphenol but because of early found positive properties of
PPO, an inexpensive source of 2,6-dimethylphenol was found and both the monomer and polymer
became commercial in 1964.
CH 3 CH 3
OH R O (4.78)
R
CH 3 CH 3
o
PPO has a very high T of 215 C, a T of about 270 C, and exhibits good hydrolytic stability,
o
g
m
but it has a very high melt viscosity and a tendency to oxidize and gel at processing temperatures
(Table 4.9). In spite of these negative processing features, PPO showed good compatibility with
polystyrene and so has found a place in the marketplace. While the methyl groups discouraged
good interactions between PPO chains, the aromatic character positively interacts with the phenyl
group on the polystyrene and the methyl groups interact positively with the aliphatic polysty-
rene backbone. The Noryl trade name covers a variety of related PPOs. PPO resins are the most
important materials for forming blends and alloys with polystyrene and styrene derivatives. These
blends and alloys with polystyrene raise the heat distortion temperature to over 100 C allowing
o
production of materials that can be boiled. Combinations with PS are more easily processed and
the PPO imparts needed fl ame resistance. The combinations also offer good hydrolytic stabilities
and electrical properties, and they are relatively light weight. They can also be modifi ed by addi-
tion to glass and other mineral fillers and are especially adaptable to metallizing. PPO-extruded
sheet is being used for solar energy collectors, lifeguards on broadcasting towers, airline beverage
cases, and window frames.
9/14/2010 3:38:26 PM
K10478.indb 126
K10478.indb 126 9/14/2010 3:38:26 PM