Page 165 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 165
128 Carraher’s Polymer Chemistry
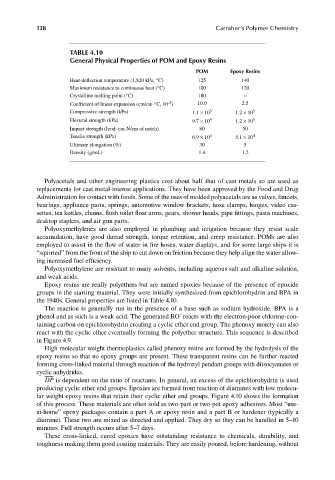
TABLE 4.10
General Physical Properties of POM and Epoxy Resins
POM Epoxy Resins
Heat-deflection temperature (1,820 kPa; °C) 125 140
Maximum resistance to continuous heat (°C) 100 120
Crystalline melting point (°C) 180 --
–5
Coefficient of linear expansion (cm/cm-°C, 10 ) 10.0 2.5
Compressive strength (kPa) 1.1 × 10 5 1.2 × 10 5
Flexural strength (kPa) 9.7 × 10 4 1.2 × 10 5
Impact strength (Izod: cm-N/cm of notch) 80 50
Tensile strength (kPa) 6.9 × 10 4 5.1 × 10 4
Ultimate elongation (%) 30 5
Density (g/mL) 1.4 1.2
Polyacetals and other engineering plastics cost about half that of cast metals so are used as
replacements for cast metal-intense applications. They have been approved by the Food and Drug
Administration for contact with foods. Some of the uses of molded polyacetals are as valves, faucets,
bearings, appliance parts, springs, automotive window brackets, hose clamps, hinges, video cas-
settes, tea kettles, chains, flush toilet float arms, gears, shower heads, pipe fittings, pasta machines,
desktop staplers, and air gun parts.
Polyoxymethylenes are also employed in plumbing and irrigation because they resist scale
accumulation, have good thread strength, torque retention, and creep resistance. POMs are also
employed to assist in the flow of water in fire hoses, water displays, and for some large ships it is
“squirted” from the front of the ship to cut down on friction because they help align the water allow-
ing increased fuel effi ciency.
Polyoxymethylene are resistant to many solvents, including aqueous salt and alkaline solution,
and weak acids.
Epoxy resins are really polyethers but are named epoxies because of the presence of epoxide
groups in the starting material. They were initially synthesized from epichlorohydrin and BPA in
the 1940s. General properties are listed in Table 4.10.
The reaction is generally run in the presence of a base such as sodium hydroxide. BPA is a
–
phenol and as such is a weak acid. The generated RO reacts with the electron-poor chlorine-con-
taining carbon on epichlorohydrin creating a cyclic ether end group. The phenoxy moiety can also
react with the cyclic ether eventually forming the polyether structure. This sequence is described
in Figure 4.9.
High molecular weight thermoplastics called phenoxy resins are formed by the hydrolysis of the
epoxy resins so that no epoxy groups are present. These transparent resins can be further reacted
forming cross-linked material through reaction of the hydroxyl pendant groups with diisocyanates or
cyclic anhydrides.
DP is dependent on the ratio of reactants. In general, an excess of the epichlorohydrin is used
producing cyclic ether end groups. Epoxies are formed from reaction of diamines with low molecu-
lar weight epoxy resins that retain their cyclic ether end groups. Figure 4.10 shows the formation
of this process. These materials are often sold as two-part or two-pot epoxy adhesives. Most “use-
at-home” epoxy packages contain a part A or epoxy resin and a part B or hardener (typically a
diamine). These two are mixed as directed and applied. They dry so they can be handled in 5–10
minutes. Full strength occurs after 5–7 days.
These cross-linked, cured epoxies have outstanding resistance to chemicals, durability, and
toughness making them good coating materials. They are easily poured, before hardening, without
9/14/2010 3:38:27 PM
K10478.indb 128
K10478.indb 128 9/14/2010 3:38:27 PM