Page 162 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 162
Polycondensation Polymers 125
Notice the tough surface and semiflexible underbelly of the dash. The amount of “foam” formation
is controlled to give the fi nished product.
PUs are also widely used as coating materials sold as finished polymers, two-part systems, and
prepolymer systems. Water-based PU systems are now available allowing easy home use. Aromatic
diisocyanate-derived coatings generally offer poor external light stability while aliphatic-derived
systems offer good light stability.
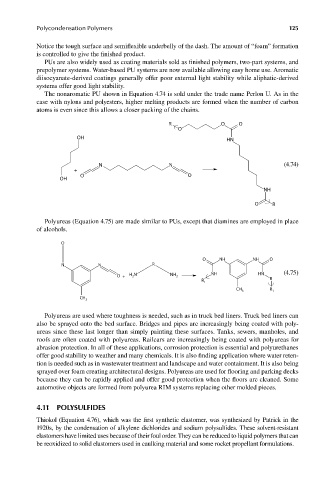
The nonaromatic PU shown in Equation 4.74 is sold under the trade name Perlon U. As in the
case with nylons and polyesters, higher melting products are formed when the number of carbon
atoms is even since this allows a closer packing of the chains.
R O O
O
OH HN
N N (4.74)
+
O O
OH
NH
O R
Polyureas (Equation 4.75) are made similar to PUs, except that diamines are employed in place
of alcohols.
O
O NH NH O
N N R
O + H N NH 2 NH HN (4.75)
2
R R
1
CH 3 R 1
CH 3
Polyureas are used where toughness is needed, such as in truck bed liners. Truck bed liners can
also be sprayed onto the bed surface. Bridges and pipes are increasingly being coated with poly-
ureas since these last longer than simply painting these surfaces. Tanks, sewers, manholes, and
roofs are often coated with polyureas. Railcars are increasingly being coated with polyureas for
abrasion protection. In all of these applications, corrosion protection is essential and polyurethanes
offer good stability to weather and many chemicals. It is also finding application where water reten-
tion is needed such as in wastewater treatment and landscape and water containment. It is also being
sprayed over foam creating architectural designs. Polyureas are used for flooring and parking decks
because they can be rapidly applied and offer good protection when the floors are cleaned. Some
automotive objects are formed from polyurea RIM systems replacing other molded pieces.
4.11 POLYSULFIDES
Thiokol (Equation 4.76), which was the first synthetic elastomer, was synthesized by Patrick in the
1920s, by the condensation of alkylene dichlorides and sodium polysulfides. These solvent-resistant
elastomers have limited uses because of their foul order. They can be reduced to liquid polymers that can
be reoxidized to solid elastomers used in caulking material and some rocket propellant formulations.
9/14/2010 3:38:26 PM
K10478.indb 125 9/14/2010 3:38:26 PM
K10478.indb 125