Page 166 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 166
Polycondensation Polymers 129
O
−
O
+
Na
CH 3
Cl O
CH
H C 3
3
− O
+ O
Na +
H 3 C Na
−
O
O
Cl
O
O
CH 3
H 3 C
+
Na O O
−
O
CH 3
+
C Na
H 3
O
−
O
O
O O
CH 3 H 3 C
OH
C CH
H 3 3
O O
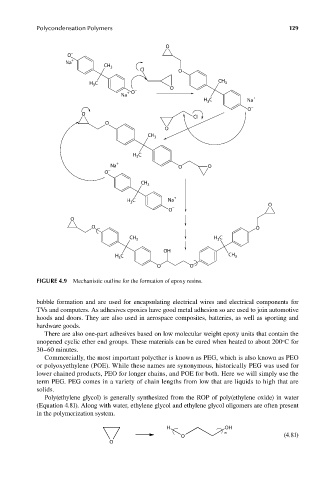
FIGURE 4.9 Mechanistic outline for the formation of epoxy resins.
bubble formation and are used for encapsulating electrical wires and electrical components for
TVs and computers. As adhesives epoxies have good metal adhesion so are used to join automotive
hoods and doors. They are also used in aerospace composites, batteries, as well as sporting and
hardware goods.
There are also one-part adhesives based on low molecular weight epoxy units that contain the
o
unopened cyclic ether end groups. These materials can be cured when heated to about 200 C for
30–60 minutes.
Commercially, the most important polyether is known as PEG, which is also known as PEO
or polyoxyethylene (POE). While these names are synonymous, historically PEG was used for
lower chained products, PEO for longer chains, and POE for both. Here we will simply use the
term PEG. PEG comes in a variety of chain lengths from low that are liquids to high that are
solids.
Poly(ethylene glycol) is generally synthesized from the ROP of poly(ethylene oxide) in water
(Equation 4.81). Along with water, ethylene glycol and ethylene glycol oligomers are often present
in the polymerization system.
H OH
n
O (4.81)
O
9/14/2010 3:38:34 PM
K10478.indb 129
K10478.indb 129 9/14/2010 3:38:34 PM