Page 171 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 171
134 Carraher’s Polymer Chemistry
OH R OH R
O
+ (4.88)
H H
CH 3 CH 3
Since the acid condensation of one mol of phenol with 1.5 mol of formaldehyde produced thermo-
set C-stage products, Baekeland reduced the relative amount of formaldehyde used and made useful
novolac resins in a two-step process. Thus, stable A-stage novolac resin is produced by heating one mol
of phenol with 0.8 mol of formaldehyde in the presence of acid. After the removal of water by vacuum
distillation, the A-stage resin produced is cooled and then pulverized. The additional formaldehyde
required to convert this linear polymer into a thermoset resin is supplied by hexamethylenetetramine.
The latter, which is admixed with the pulverized A-stage resin, is produced by the condensation of
formaldehyde and ammonia. Other ingredients such as filler, pigments, and lubricants are mixed in
with the resin and hexamethylenetetramine. The A-stage resin is further polymerized. The term phe-
nol molding compound is applied to the granulated B-stage novolac resin.
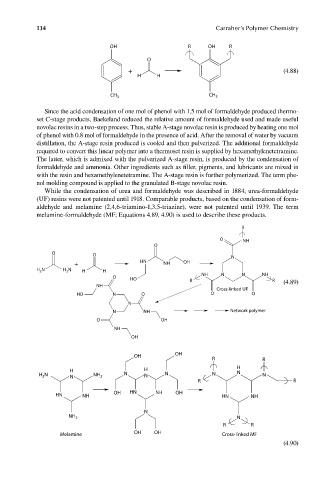
While the condensation of urea and formaldehyde was described in 1884, urea-formaldehyde
(UF) resins were not patented until 1918. Comparable products, based on the condensation of form-
aldehyde and melamine (2,4,6-triamino-1,3,5-triazine), were not patented until 1939. The term
melamine-formaldehyde (MF; Equations 4.89, 4.90) is used to describe these products.
R
O NH
O
O O
N
+ HN NH OH
H 2 N H 2 N H H
NH N N NH
O HO R R
NH (4.89)
Cross-linked UF
HO N O O O
N
N NH Network polymer
O OH
NH
OH
OH OH
R R
H H H
N NH N N N N N
H 2 N
N 2
R R
OH HN NH OH
HN NH HN NH
N
NH 2 N
R R
Melamine OH OH Cross-linked MF
(4.90)
9/14/2010 3:38:35 PM
K10478.indb 134
K10478.indb 134 9/14/2010 3:38:35 PM