Page 172 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 172
Polycondensation Polymers 135
O O
O O
O
O
OH
O
O
R
R
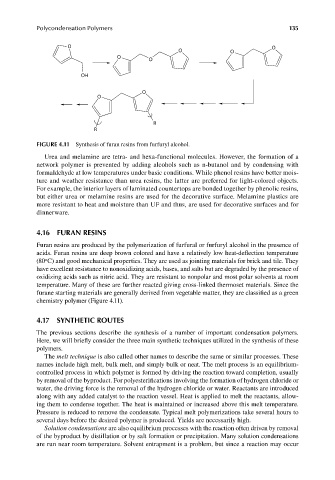
FIGURE 4.11 Synthesis of furan resins from furfuryl alcohol.
Urea and melamine are tetra- and hexa-functional molecules. However, the formation of a
network polymer is prevented by adding alcohols such as n-butanol and by condensing with
formaldehyde at low temperatures under basic conditions. While phenol resins have better mois-
ture and weather resistance than urea resins, the latter are preferred for light-colored objects.
For example, the interior layers of laminated countertops are bonded together by phenolic resins,
but either urea or melamine resins are used for the decorative surface. Melamine plastics are
more resistant to heat and moisture than UF and thus, are used for decorative surfaces and for
dinnerware.
4.16 FURAN RESINS
Furan resins are produced by the polymerization of furfural or furfuryl alcohol in the presence of
acids. Furan resins are deep brown colored and have a relatively low heat-defl ection temperature
(80 C) and good mechanical properties. They are used as jointing materials for brick and tile. They
o
have excellent resistance to nonoxidizing acids, bases, and salts but are degraded by the presence of
oxidizing acids such as nitric acid. They are resistant to nonpolar and most polar solvents at room
temperature. Many of these are further reacted giving cross-linked thermoset materials. Since the
furane starting materials are generally derived from vegetable matter, they are classified as a green
chemistry polymer (Figure 4.11).
4.17 SYNTHETIC ROUTES
The previous sections describe the synthesis of a number of important condensation polymers.
Here, we will briefly consider the three main synthetic techniques utilized in the synthesis of these
polymers.
The melt technique is also called other names to describe the same or similar processes. These
names include high melt, bulk melt, and simply bulk or neat. The melt process is an equilibrium-
controlled process in which polymer is formed by driving the reaction toward completion, usually
by removal of the byproduct. For polyesterifications involving the formation of hydrogen chloride or
water, the driving force is the removal of the hydrogen chloride or water. Reactants are introduced
along with any added catalyst to the reaction vessel. Heat is applied to melt the reactants, allow-
ing them to condense together. The heat is maintained or increased above this melt temperature.
Pressure is reduced to remove the condensate. Typical melt polymerizations take several hours to
several days before the desired polymer is produced. Yields are necessarily high.
Solution condensations are also equilibrium processes with the reaction often driven by removal
of the byproduct by distillation or by salt formation or precipitation. Many solution condensations
are run near room temperature. Solvent entrapment is a problem, but since a reaction may occur
9/14/2010 3:38:36 PM
K10478.indb 135 9/14/2010 3:38:36 PM
K10478.indb 135