Page 169 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 169
132 Carraher’s Polymer Chemistry
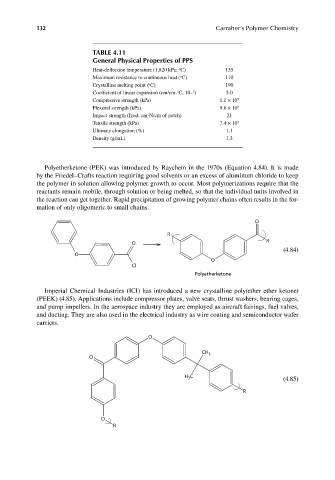
TABLE 4.11
General Physical Properties of PPS
Heat-deflection temperature (1,820 kPa; C) 135
o
o
Maximum resistance to continuous heat ( C) 110
Crystalline melting point ( C) 190
o
5
o
Coefficient of linear expansion (cm/cm- C, 10– ) 5.0
Compressive strength (kPa) 1.1 × 10 5
Flexural strength (kPa) 9.6 × 10 5
Impact strength (Izod: cm-N/cm of notch) 21
Tensile strength (kPa) 7.4 × 10 4
Ultimate elongation (%) 1.1
Density (g/mL) 1.3
Polyetherketone (PEK) was introduced by Raychem in the 1970s (Equation 4.84). It is made
by the Friedel–Crafts reaction requiring good solvents or an excess of aluminum chloride to keep
the polymer in solution allowing polymer growth to occur. Most polymerizations require that the
reactants remain mobile, through solution or being melted, so that the individual units involved in
the reaction can get together. Rapid precipitation of growing polymer chains often results in the for-
mation of only oligomeric to small chains.
O
R
R
O
(4.84)
O
O
Cl
Polyetherketone
Imperial Chemical Industries (ICI) has introduced a new crystalline poly(ether ether ketone)
(PEEK) (4.85). Applications include compressor plates, valve seats, thrust washers, bearing cages,
and pump impellers. In the aerospace industry they are employed as aircraft fairings, fuel valves,
and ducting. They are also used in the electrical industry as wire coating and semiconductor wafer
carriers.
O
CH 3
O
H C (4.85)
3
R
O
R
9/14/2010 3:38:35 PM
K10478.indb 132
K10478.indb 132 9/14/2010 3:38:35 PM