Page 603 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 603
566 Carraher’s Polymer Chemistry
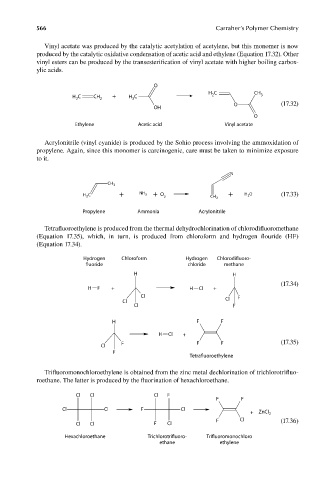
Vinyl acetate was produced by the catalytic acetylation of acetylene, but this monomer is now
produced by the catalytic oxidative condensation of acetic acid and ethylene (Equation 17.32). Other
vinyl esters can be produced by the transesterifi cation of vinyl acetate with higher boiling carbox-
ylic acids.
O
H C CH
H C CH 2 + H C 2 3
3
2
O (17.32)
OH
O
Ethylene Acetic acid Vinyl acetate
Acrylonitrile (vinyl cyanide) is produced by the Sohio process involving the ammoxidation of
propylene. Again, since this monomer is carcinogenic, care must be taken to minimize exposure
to it.
N
CH 3
HC + NH 3 + O 2 CH 2 + HO (17.33)
2
2
Propylene Ammonia Acrylonitrile
Tetrafluoroethylene is produced from the thermal dehydrochlorination of chlorodifl uoromethane
(Equation 17.35), which, in turn, is produced from chloroform and hydrogen fl ouride (HF)
(Equation 17.34).
Hydrogen Chloroform Hydrogen Chlorodifluoro-
fluoride chloride methane
H H
(17.34)
H F + H Cl +
Cl F
Cl Cl
Cl F
H F F
H Cl +
F F F (17.35)
Cl
F
Tetrafluoroethylene
Trifluoromonochloroethylene is obtained from the zinc metal dechlorination of trichlorotrifl uo-
roethane. The latter is produced by the fluorination of hexachloroethane.
Cl Cl Cl F
F F
Cl Cl F Cl
+ ZnCl 2
F Cl (17.36)
Cl Cl F Cl
Hexachloroethane Trichlorotrifluoro- Trifluoromonochloro
ethane ethylene
9/14/2010 3:43:26 PM
K10478.indb 566 9/14/2010 3:43:26 PM
K10478.indb 566