Page 607 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 607
570 Carraher’s Polymer Chemistry
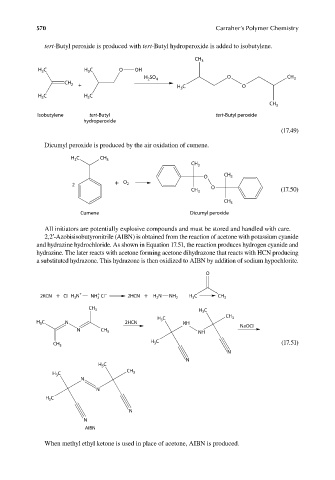
tert-Butyl peroxide is produced with tert-Butyl hydroperoxide is added to isobutylene.
CH 3
H C H C O OH
3
3
H SO 4 O CH 3
2
CH 2 +
H C O
3
H 3 C H 3 C
CH 3
Isobutylene tert-Butyl tert-Butyl peroxide
hydroperoxide
(17.49)
Dicumyl peroxide is produced by the air oxidation of cumene.
H C CH 3
3
CH 3
O CH 3
+ O 2
2
CH 3 O (17.50)
CH 3
Cumene Dicumyl peroxide
All initiators are potentially explosive compounds and must be stored and handled with care.
2,2′-Azobisisobutyronitrile (AIBN) is obtained from the reaction of acetone with potassium cyanide
and hydrazine hydrochloride. As shown in Equation 17.51, the reaction produces hydrogen cyanide and
hydrazine. The later reacts with acetone forming acetone dihydrazone that reacts with HCN producing
a substituted hydrazone. This hydrazone is then oxidized to AIBN by addition of sodium hypochlorite.
O
−
2KCN + Cl H 3 N + NH 3 Cl − 2HCN + H 2 N NH 2 H 3 C CH 3
+
CH 3 H 3 C
H 3 C CH 3
H 3 C N 2HCN NH
NaOCl
N CH 3 NH
H 3 C (17.51)
CH 3
N
N
H 3 C
H 3 C CH 3
N
N
H 3 C
N
N
AIBN
When methyl ethyl ketone is used in place of acetone, AIBN is produced.
9/14/2010 3:43:32 PM
K10478.indb 570 9/14/2010 3:43:32 PM
K10478.indb 570