Page 605 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 605
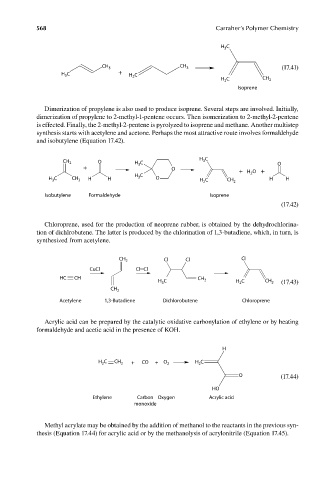
568 Carraher’s Polymer Chemistry
H C
3
CH
CH 3 3 (17.41)
H C + H C
3
2
H 2 C CH 2
Isoprene
Dimerization of propylene is also used to produce isoprene. Several steps are involved. Initially,
dimerization of propylene to 2-methyl-1-pentene occurs. Then isomerization to 2-methyl-2-pentene
is effected. Finally, the 2-methyl-2-pentene is pyrolyzed to isoprene and methane. Another multistep
synthesis starts with acetylene and acetone. Perhaps the most attractive route involves formaldehyde
and isobutylene (Equation 17.42).
H C
CH 2 O H C 3 O
3
+ O + H O +
2
H C CH 3 H H H 3 C O H 2 C CH 2 H H
3
Isobutylene Formaldehyde Isoprene
(17.42)
Chloroprene, used for the production of neoprene rubber, is obtained by the dehydrochlorina-
tion of dichlrobutene. The latter is produced by the chlorination of 1,3-butadiene, which, in turn, is
synthesized from acetylene.
CH 2 Cl Cl Cl
CuCl Cl Cl
HC CH
H C CH 2 H C CH 2 (17.43)
3
2
CH 2
Acetylene 1,3-Butadiene Dichlorobutene Chloroprene
Acrylic acid can be prepared by the catalytic oxidative carbonylation of ethylene or by heating
formaldehyde and acetic acid in the presence of KOH.
H
H C CH 2 + CO + O 2 H C
2
2
O (17.44)
HO
Ethylene Carbon Oxygen Acrylic acid
monoxide
Methyl acrylate may be obtained by the addition of methanol to the reactants in the previous syn-
thesis (Equation 17.44) for acrylic acid or by the methanolysis of acrylonitrile (Equation 17.45).
9/14/2010 3:43:30 PM
K10478.indb 568 9/14/2010 3:43:30 PM
K10478.indb 568