Page 604 - Carrahers_Polymer_Chemistry,_Eighth_Edition
P. 604
Synthesis of Reactants and Intermediates for Polymers 567
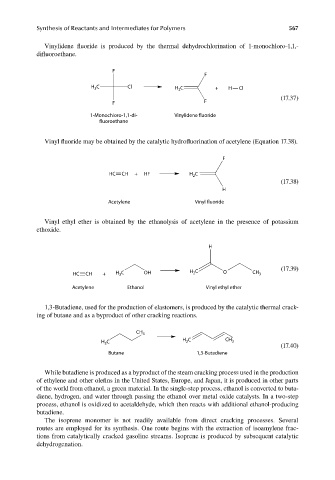
Vinylidene fluoride is produced by the thermal dehydrochlorination of 1-monochloro-1,1,-
difl uoroethane.
F
F
H C Cl H C + H Cl
3
2
(17.37)
F F
1-Monochloro-1,1-di- Vinylidene fluoride
fluoroethane
Vinyl fluoride may be obtained by the catalytic hydrofluorination of acetylene (Equation 17.38).
F
HC CH + HF H C
2
(17.38)
H
Acetylene Vinyl fluoride
Vinyl ethyl ether is obtained by the ethanolysis of acetylene in the presence of potassium
ethoxide.
H
(17.39)
H C
HC CH + H C OH 2 O CH 3
3
Acetylene Ethanol Vinyl ethyl ether
1,3-Butadiene, used for the production of elastomers, is produced by the catalytic thermal crack-
ing of butane and as a byproduct of other cracking reactions.
CH 3
H C
H C 2 CH 3
3
(17.40)
Butane 1,3-Butadiene
While butadiene is produced as a byproduct of the steam cracking process used in the production
of ethylene and other olefi ns in the United States, Europe, and Japan, it is produced in other parts
of the world from ethanol, a green material. In the single-step process, ethanol is converted to buta-
diene, hydrogen, and water through passing the ethanol over metal oxide catalysts. In a two-step
process, ethanol is oxidized to acetaldehyde, which then reacts with additional ethanol-producing
butadiene.
The isoprene monomer is not readily available from direct cracking processes. Several
routes are employed for its synthesis. One route begins with the extraction of isoamylene frac-
tions from catalytically cracked gasoline streams. Isoprene is produced by subsequent catalytic
dehydrogenation.
9/14/2010 3:43:29 PM
K10478.indb 567
K10478.indb 567 9/14/2010 3:43:29 PM