Page 23 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 23
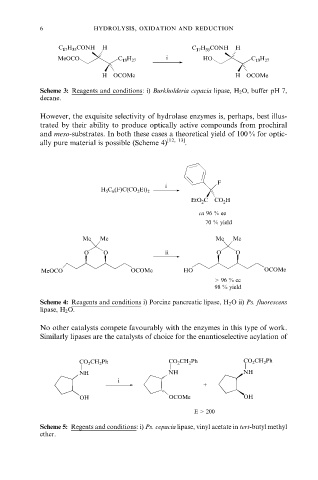
6 hydrolysis, oxidation and reduction
C 17 H 35 CONH H C 17 H 35 CONH H
MeOCO C 13 H 27 i HO C 13 H 27
H OCOMe H OCOMe
Scheme 3: Reagents and conditions: i) Burkholderia cepacia lipase, H 2 O, buffer pH 7,
decane.
However, the exquisite selectivity of hydrolase enzymes is, perhaps, best illus-
trated by their ability to produce optically active compounds from prochiral
and meso-substrates. In both these cases a theoretical yield of 100 % for optic-
ally pure material is possible (Scheme 4) [12, 13] .
F
i
H 5 C 6 (F)C(CO 2 Et) 2
EtO 2 C CO 2 H
ca 96 % ee
70 % yield
Me Me Me Me
O O ii O O
MeOCO OCOMe HO OCOMe
> 96 % ee
98 % yield
Scheme 4: Reagents and conditions i) Porcine pancreatic lipase, H 2 O ii) Ps. fluorescens
lipase, H 2 O.
No other catalysts compete favourably with the enzymes in this type of work.
Similarly lipases are the catalysts of choice for the enantioselective acylation of
CO 2 CH 2 Ph CO 2 CH 2 Ph CO 2 CH 2 Ph
NH NH NH
i
+
OH OCOMe OH
E > 200
Scheme 5: Regents and conditions: i) Ps. cepacia lipase, vinyl acetate in tert-butyl methyl
ether.