Page 24 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 24
the integration of biotransformations into catalyst 7
a wide a variety of alcohols. This area of research has mushroomed since
Klibanov's seminal studies clearly indicating that the procedure is exceedingly
simple; a comprehensive review of the methodology is available [14] . A typical
example of a resolution process involving enantioselective esterification using a
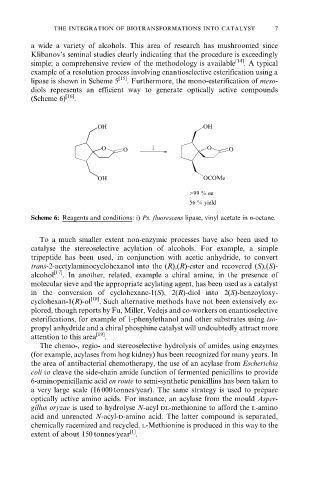
lipase is shown in Scheme 5 [15] . Furthermore, the mono-esterification of meso-
diols represents an efficient way to generate optically active compounds
(Scheme 6) [16] .
OH OH
O O i O O
OH OCOMe
>99 % ee
56 % yield
Scheme 6: Reagents and conditions: i) Ps. fluorescens lipase, vinyl acetate in n-octane.
To a much smaller extent non-enzymic processes have also been used to
catalyse the stereoselective acylation of alcohols. For example, a simple
tripeptide has been used, in conjunction with acetic anhydride, to convert
trans-2-acetylaminocyclohexanol into the (R),(R)-ester and recovered (S),(S)-
alcohol [17] . In another, related, example a chiral amine, in the presence of
molecular sieve and the appropriate acylating agent, has been used as a catalyst
in the conversion of cyclohexane-1(S), 2(R)-diol into 2(S)-benzoyloxy-
cyclohexan-1(R)-ol [18] . Such alternative methods have not been extensively ex-
plored, though reports by Fu, Miller, Vedejs and co-workers on enantioselective
esterifications, for example of 1-phenylethanol and other substrates using iso-
propyl anhydride and a chiral phosphine catalyst will undoubtedly attract more
attention to this area [19] .
The chemo-, regio- and stereoselective hydrolysis of amides using enzymes
(for example, acylases from hog kidney) has been recognized for many years. In
the area of antibacterial chemotherapy, the use of an acylase from Escherichia
coli to cleave the side-chain amide function of fermented penicillins to provide
6-aminopenicillanic acid en route to semi-synthetic penicillins has been taken to
a very large scale (16 000 tonnes/year). The same strategy is used to prepare
optically active amino acids. For instance, an acylase from the mould Asper-
gillus oryzae is used to hydrolyse N-acyl dl-methionine to afford the l-amino
acid and unreacted N-acyl-d-amino acid. The latter compound is separated,
chemically racemized and recycled. l-Methionine is produced in this way to the
[1]
extent of about 150 tonnes/year .