Page 25 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 25
8 hydrolysis, oxidation and reduction
The hydrolysis of racemic non-natural amides has led to useful products and
intermediates for the fine chemical industry. Thus hydrolysis of the racemic
amide (2) with an acylase in Rhodococcus erythrolpolis furnished the (S)-acid
(the anti-inflammatory agent Naproxen) in 42 % yield and > 99 % enantiomeric
excess [20] . Obtaining the g-lactam (ÿ)-(3) has been the subject of much research
and development effort, since the compound is a very versatile synthon for the
production of carbocyclic nucleosides. An acylase from Comamonas acidovor-
ans has been isolated, cloned and overexpressed. The acylase tolerates a 500 g/
litre input of racemic lactam, hydrolyses only the ()-enantiomer leaving the
desired intermediate essentially optically pure (E > 400) [21] .
Me
NH
CONH 2
MeO O
(2) (−)-(3)
The enzyme-catalysed hydrolysis of epoxides has been reviewed [22] . Much of
the early work featured liver microsomal epoxide hydrolases but the very nature
and origin of these biocatalysts meant that they would always be limited to the
small scale. In recent years the use of epoxide-hydrolase enzymes within organ-
isms has become popular, with the fungus Beauvaria sulfurescens being featured
regularly. For instance, incubation of styrene oxide with this organism provides
(R)-1-phenylethanediol (45 % yield; 83 % ee) and recovered (R)-styrene oxide
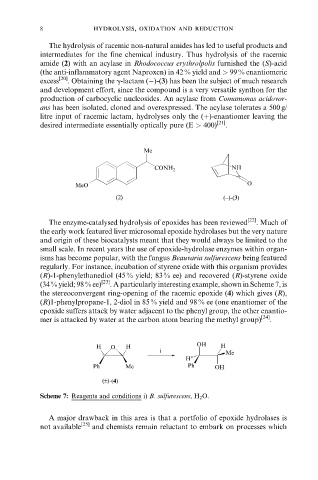
(34 % yield; 98 % ee) [23] . A particularly interesting example, shown in Scheme 7, is
the stereoconvergent ring-opening of the racemic epoxide (4) which gives (R),
(R)1-phenylpropane-1, 2-diol in 85 % yield and 98 % ee (one enantiomer of the
epoxide suffers attack by water adjacent to the phenyl group, the other enantio-
mer is attacked by water at the carbon atom bearing the methyl group) [24] .
H O H OH H
i Me
H
Ph Me Ph OH
(±)-(4)
Scheme 7: Reagents and conditions i) B. sulfurescens, H 2 O.
A major drawback in this area is that a portfolio of epoxide hydrolases is
not available [25] and chemists remain reluctant to embark on processes which