Page 26 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 26
the integration of biotransformations into catalyst 9
involve the use of whole cells (such as B. sulfurescens). Not surprisingly, there-
fore, the use of a non-enzymic method for the kinetic resolution of terminal
epoxides and the stereoselective opening of meso-epoxides, involving salen±
cobalt complexes, has aroused interest. For example, use of the organometallic
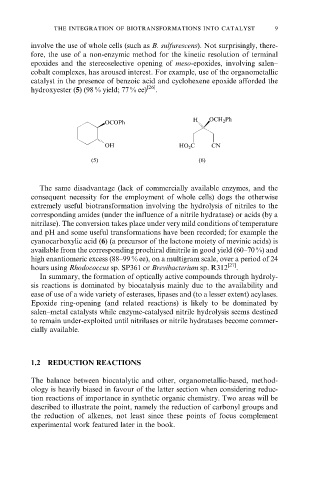
catalyst in the presence of benzoic acid and cyclohexene epoxide afforded the
hydroxyester (5) (98 % yield; 77 % ee) [26] .
H OCH 2 Ph
OCOPh
OH HO 2 C CN
(5) (6)
The same disadvantage (lack of commercially available enzymes, and the
consequent necessity for the employment of whole cells) dogs the otherwise
extremely useful biotransformation involving the hydrolysis of nitriles to the
corresponding amides (under the influence of a nitrile hydratase) or acids (by a
nitrilase). The conversion takes place under very mild conditions of temperature
and pH and some useful transformations have been recorded; for example the
cyanocarboxylic acid (6) (a precursor of the lactone moiety of mevinic acids) is
available from the corresponding prochiral dinitrile in good yield (60±70 %) and
high enantiomeric excess (88±99 % ee), on a multigram scale, over a period of 24
hours using Rhodococcus sp. SP361 or Brevibacterium sp. R312 [27] .
In summary, the formation of optically active compounds through hydroly-
sis reactions is dominated by biocatalysis mainly due to the availability and
ease of use of a wide variety of esterases, lipases and (to a lesser extent) acylases.
Epoxide ring-opening (and related reactions) is likely to be dominated by
salen±metal catalysts while enzyme-catalysed nitrile hydrolysis seems destined
to remain under-exploited until nitrilases or nitrile hydratases become commer-
cially available.
1.2 REDUCTION REACTIONS
The balance between biocatalytic and other, organometallic-based, method-
ology is heavily biased in favour of the latter section when considering reduc-
tion reactions of importance in synthetic organic chemistry. Two areas will be
described to illustrate the point, namely the reduction of carbonyl groups and
the reduction of alkenes, not least since these points of focus complement
experimental work featured later in the book.