Page 31 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 31
14 hydrolysis, oxidation and reduction
One facet of the whole cell work that draws attention is the sometimes
profitable operation of a cascade of reactions in the multi-enzyme portfolio of
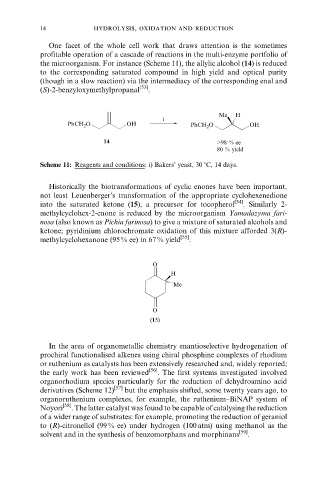
the microorganism. For instance (Scheme 11), the allylic alcohol (14) is reduced
to the corresponding saturated compound in high yield and optical purity
(though in a slow reaction) via the intermediacy of the corresponding enal and
(S)-2-benzyloxymethylpropanal [53] .
Me H
i
PhCH 2 O OH PhCH 2 O OH
14 >98 % ee
80 % yield
Scheme 11: Reagents and conditions: i) Bakers' yeast, 30 8C, 14 days.
Historically the biotransformations of cyclic enones have been important,
not least Leuenberger's transformation of the appropriate cyclohexenedione
into the saturated ketone (15), a precursor for tocopherol [54] . Similarly 2-
methylcyclohex-2-enone is reduced by the microorganism Yamadazyma fari-
nosa (also known as Pichia farinosa) to give a mixture of saturated alcohols and
ketone; pyridinium chlorochromate oxidation of this mixture afforded 3(R)-
methylcyclohexanone (95 % ee) in 67 % yield [55] .
O
H
Me
O
(15)
In the area of organometallic chemistry enantioselective hydrogenation of
prochiral functionalised alkenes using chiral phosphine complexes of rhodium
or ruthenium as catalysts has been extensively researched and, widely reported;
the early work has been reviewed [56] . The first systems investigated involved
organorhodium species particularly for the reduction of dehydroamino acid
derivatives (Scheme 12) [57] but the emphasis shifted, some twenty years ago, to
organoruthenium complexes, for example, the ruthenium±BiNAP system of
[58]
Noyori . The latter catalyst was found to be capable of catalysing the reduction
of a wider range of substrates: for example, promoting the reduction of geraniol
to (R)-citronellol (99 % ee) under hydrogen (100 atm) using methanol as the
solvent and in the synthesis of benzomorphans and morphinans [59] .