Page 35 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 35
18 hydrolysis, oxidation and reduction
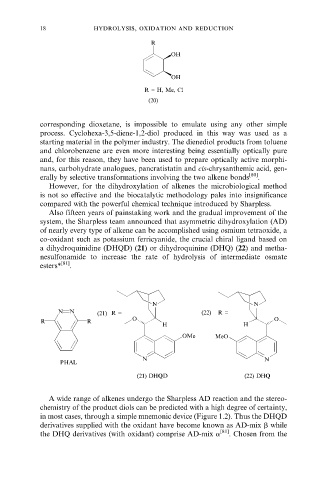
R
OH
OH
R = H, Me, Cl
(20)
corresponding dioxetane, is impossible to emulate using any other simple
process. Cyclohexa-3,5-diene-1,2-diol produced in this way was used as a
starting material in the polymer industry. The dienediol products from toluene
and chlorobenzene are even more interesting being essentially optically pure
and, for this reason, they have been used to prepare optically active morphi-
nans, carbohydrate analogues, pancratistatin and cis-chrysanthemic acid, gen-
erally by selective transformations involving the two alkene bonds [80] .
However, for the dihydroxylation of alkenes the microbiological method
is not so effective and the biocatalytic methodology pales into insignificance
compared with the powerful chemical technique introduced by Sharpless.
Also fifteen years of painstaking work and the gradual improvement of the
system, the Sharpless team announced that asymmetric dihydroxylation (AD)
of nearly every type of alkene can be accomplished using osmium tetraoxide, a
co-oxidant such as potassium ferricyanide, the crucial chiral ligand based on
a dihydroquinidine (DHQD) (21) or dihydroquinine (DHQ) (22) and metha-
nesulfonamide to increase the rate of hydrolysis of intermediate osmate
esters* [81] .
N N
N N (21) R = (22) R =
R R O O
H H
OMe MeO
N N
PHAL
(21) DHQD (22) DHQ
A wide range of alkenes undergo the Sharpless AD reaction and the stereo-
chemistry of the product diols can be predicted with a high degree of certainty,
in most cases, through a simple mnemonic device (Figure 1.2). Thus the DHQD
derivatives supplied with the oxidant have become known as AD-mix b while
the DHQ derivatives (with oxidant) comprise AD-mix a [81] . Chosen from the