Page 40 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 40
the integration of biotransformations into catalyst 23
new methods. For example, Enders has shown that oxygen in the presence of
diethylzinc and N-methyl ephedrine converts enones into epoxides in excellent
yields and very good enantiomeric excesses* (up to 92 %) [96] . Alternatively,
Jackson et al. have reported the employment of tert-butyl hydroperoxide as
the oxidant together with catalytic amounts of dibutyl magnesium and diethyl
tartrate. Chalcones are oxidized to the corresponding epoxides under these
conditions in yields varying between 40±60 % and good to excellent enantio-
meric excess [97] .
For a similar series of chalcone derivatives the use of aqueous sodium
hypochlorite in a two phase system (with toluene as the organic solvent) and
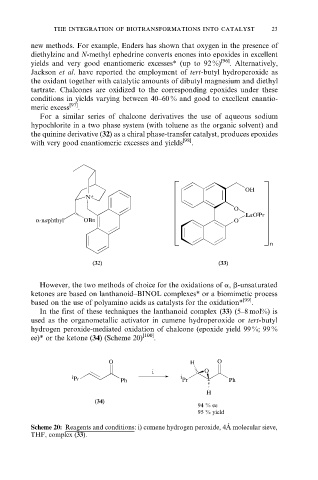
the quinine derivative (32) as a chiral phase-transfer catalyst, produces epoxides
with very good enantiomeric excesses and yields [98] .
OH
N +
O
La O i Pr
α-naphthyl OBn O
n
(32) (33)
However, the two methods of choice for the oxidations of a, b-unsaturated
ketones are based on lanthanoid±BINOL complexes* or a biomimetic process
based on the use of polyamino acids as catalysts for the oxidation* [99] .
In the first of these techniques the lanthanoid complex (33) (5±8 mol%) is
used as the organometallic activator in cumene hydroperoxide or tert-butyl
hydrogen peroxide-mediated oxidation of chalcone (epoxide yield 99 %; 99 %
ee)* or the ketone (34) (Scheme 20) [100] .
O H O
i O
i
i Pr Pr Ph
Ph
H
(34)
94 % ee
95 % yield
Ê
Scheme 20: Reagents and conditions: i) cumene hydrogen peroxide, 4A molecular sieve,
THF, complex (33).