Page 39 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 39
22 hydrolysis, oxidation and reduction
The asymmetric epoxidation of E-alkenes and terminal alkenes proved to be
more difficult, though a recent finding, describing the use of a modified salen
complex to epoxidize (E)-b-methylstyrene to form the corresponding epoxide
in 83 % ee, represents another important step forward. Alternatively, chiral
(D 2 -symmetric) porphyrins have been used, in conjunction with ruthenium* or
iron, for efficient asymmetric oxidation of trans- and terminal alkenes [92] .
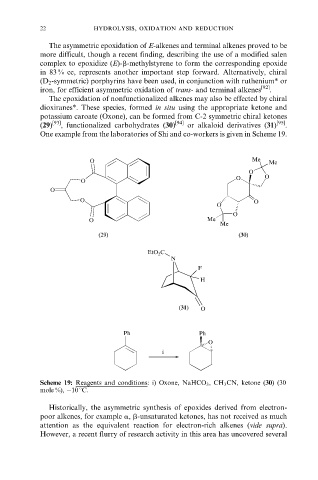
The epoxidation of nonfunctionalized alkenes may also be effected by chiral
dioxiranes*. These species, formed in situ using the appropriate ketone and
potassium caroate (Oxone), can be formed from C-2 symmetric chiral ketones
(29) [93] , functionalized carbohydrates (30) [94] or alkaloid derivatives (31) [95] .
One example from the laboratories of Shi and co-workers is given in Scheme 19.
O Me Me
O
O O
O
O
O O
O
O
O Me
Me
(29) (30)
EtO 2 C
N
F
H
(31) O
Ph Ph
O
i
Scheme 19: Reagents and conditions: i) Oxone, NaHCO 3 , CH 3 CN, ketone (30) (30
mole %), ÿ10 8C.
Historically, the asymmetric synthesis of epoxides derived from electron-
poor alkenes, for example a, b-unsaturated ketones, has not received as much
attention as the equivalent reaction for electron-rich alkenes (vide supra).
However, a recent flurry of research activity in this area has uncovered several