Page 43 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 43
26 hydrolysis, oxidation and reduction
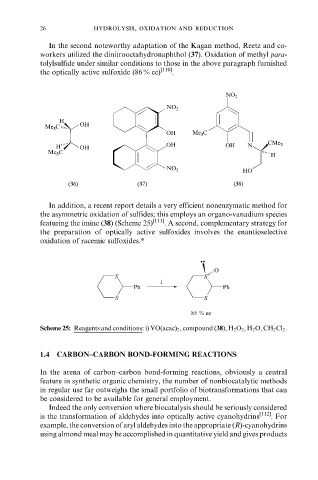
In the second noteworthy adaptation of the Kagan method, Reetz and co-
workers utilized the dinitrooctahydronaphthol (37). Oxidation of methyl para-
tolylsulfide under similar conditions to those in the above paragraph furnished
the optically active sulfoxide (86 % ee) [110] .
NO 2
NO 2
H
Me 3 C OH
OH Me 3 C
H OH OH OH N CMe 3
Me 3 C
H
NO 2
HO
(36) (37) (38)
In addition, a recent report details a very efficient nonenzymatic method for
the asymmetric oxidation of sulfides; this employs an organo-vanadium species
featuring the imine (38) (Scheme 25) [111] . A second, complementary strategy for
the preparation of optically active sulfoxides involves the enantioselective
oxidation of racemic sulfoxides.*
O
S S
i
Ph Ph
S S
85 % ee
Scheme 25: Reagents and conditions: i) VO(acac) 2 , compound (38), H 2 O 2 , H 2 O, CH 2 Cl 2 .
1.4 CARBON±CARBON BOND-FORMING REACTIONS
In the arena of carbon±carbon bond-forming reactions, obviously a central
feature in synthetic organic chemistry, the number of nonbiocatalytic methods
in regular use far outweighs the small portfolio of biotransformations that can
be considered to be available for general employment.
Indeed the only conversion where biocatalysis should be seriously considered
is the transformation of aldehydes into optically active cyanohydrins [112] . For
example, the conversion of aryl aldehydes into the appropriate (R)-cyanohydrins
using almond meal may be accomplished in quantitative yield and gives products