Page 46 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 46
the integration of biotransformations into catalyst 29
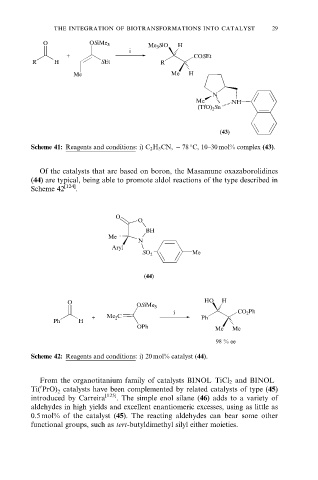
O OSiMe 3 Me 3 SiO H
i
+ COSEt
R H SEt R
Me Me H
N
Me NH
(TfO) 2 Sn
(43)
Scheme 41: Reagents and conditions: i) C 2 H 5 CN, ÿ 78 C, 10±30 mol% complex (43).
Of the catalysts that are based on boron, the Masamune oxazaborolidines
(44) are typical, being able to promote aldol reactions of the type described in
Scheme 42 [124] .
O
O
BH
Me
N
Aryl
Me
SO 2
(44)
O HO H
OSiMe 3
i CO 2 Ph
+ Me 2 C Ph
Ph H
OPh Me Me
98 % ee
Scheme 42: Reagents and conditions: i) 20 mol% catalyst (44).
From the organotitanium family of catalysts BINOL±TiCl 2 and BINOL±
i
Ti( PrO) catalysts have been complemented by related catalysts of type (45)
2
introduced by Carreira [125] . The simple enol silane (46) adds to a variety of
aldehydes in high yields and excellent enantiomeric excesses, using as little as
0.5 mol% of the catalyst (45). The reacting aldehydes can bear some other
functional groups, such as tert-butyldimethyl silyl either moieties.