Page 51 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 51
34 hydrolysis, oxidation and reduction
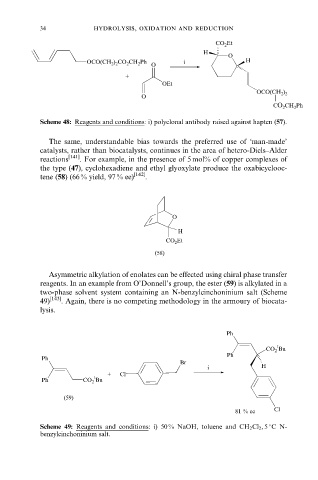
CO 2 Et
H
O
OCO(CH 2 ) 2 CO 2 CH 2 Ph i H
O
+
OEt
OCO(CH 2 ) 2
O
CO 2 CH 2 Ph
Scheme 48: Reagents and conditions: i) polyclonal antibody raised against hapten (57).
The same, understandable bias towards the preferred use of `man-made'
catalysts, rather than biocatalysts, continues in the area of hetero-Diels±Alder
reactions [141] . For example, in the presence of 5 mol% of copper complexes of
the type (47), cyclohexadiene and ethyl glyoxylate produce the oxabicyclooc-
tene (58) (66 % yield, 97 % ee) [142] .
O
H
CO 2 Et
(58)
Asymmetric alkylation of enolates can be effected using chiral phase transfer
reagents. In an example from O'Donnell's group, the ester (59) is alkylated in a
two-phase solvent system containing an N-benzylcinchoninium salt (Scheme
49) [143] . Again, there is no competing methodology in the armoury of biocata-
lysis.
Ph
t
CO 2 Bu
Ph
Ph
Br
i H
+ Cl
t
Ph CO 2 Bu
(59)
81 % ee Cl
Scheme 49: Reagents and conditions: i) 50 % NaOH, toluene and CH 2 Cl 2 , 5 C N-
benzylcinchoninium salt.