Page 52 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 52
the integration of biotransformations into catalyst 35
The promotion of carbon±carbon bond forming reactions involving alkenes
is, once again, almost entirely within the domain of non-natural catalysts.
One example each from five important areas are described below. It should
be noted that this area is one of intense current interest and new catalysts
and novel methodologies are appearing monthly; thus the following selection
gives the reader only a glimpse of the important ground-breaking work in this
area.
Hydrocyanation of alkenes (and alkynes) is an efficient route to nitriles en
route to many types of fine chemicals. Initial studies of the hydrocyanation of
vinylarenes such as styrene involved the use of a nickel±DIOP system, but ee's
were disappointing at ca 10 %. More success was achieved with carbohydrate
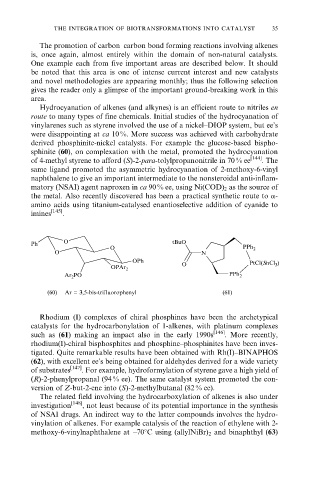
derived phosphinite-nickel catalysts. For example the glucose-based bispho-
sphinite (60), on complexation with the metal, promoted the hydrocyanation
of 4-methyl styrene to afford (S)-2-para-tolylpropanonitrile in 70 % ee [144] . The
same ligand promoted the asymmetric hydrocyanation of 2-methoxy-6-vinyl
naphthalene to give an important intermediate to the nonsteroidal anti-inflam-
matory (NSAI) agent naproxen in ca 90 % ee, using Ni(COD) 2 as the source of
the metal. Also recently discovered has been a practical synthetic route to a-
amino acids using titanium-catalysed enantioselective addition of cyanide to
imines [145] .
Ph O tBuO
O PPh 2
O N
OPh
O PtCl(SnCl 3 )
OPAr 2
Ar 2 PO PPh 2
(60) Ar = 3,5-bis-trifluorophenyl (61)
Rhodium (I) complexes of chiral phosphines have been the archetypical
catalysts for the hydrocarbonylation of 1-alkenes, with platinum complexes
such as (61) making an impact also in the early 1990s [146] . More recently,
rhodium(I)-chiral bisphosphites and phosphine±phosphinites have been inves-
tigated. Quite remarkable results have been obtained with Rh(I)±BINAPHOS
(62), with excellent ee's being obtained for aldehydes derived for a wide variety
of substrates [147] . For example, hydroformylation of styrene gave a high yield of
(R)-2-phenylpropanal (94 % ee). The same catalyst system promoted the con-
version of Z-but-2-ene into (S)-2-methylbutanal (82 % ee).
The related field involving the hydrocarboxylation of alkenes is also under
investigation [148] , not least because of its potential importance in the synthesis
of NSAI drugs. An indirect way to the latter compounds involves the hydro-
vinylation of alkenes. For example catalysis of the reaction of ethylene with 2-
methoxy-6-vinylnaphthalene at ÿ708C using (allylNiBr) 2 and binaphthyl (63)