Page 53 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 53
36 hydrolysis, oxidation and reduction
OCH 2 Ph
PPh 2
Rh(acac)
O PPh 2
P
O
O
(62) (63)
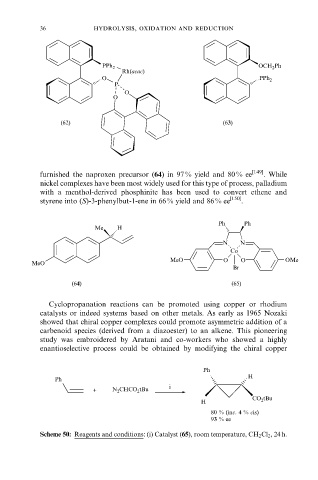
furnished the naproxen precursor (64) in 97 % yield and 80 % ee [149] . While
nickel complexes have been most widely used for this type of process, palladium
with a menthol-derived phosphinite has been used to convert ethene and
styrene into (S)-3-phenylbut-1-ene in 66 % yield and 86 % ee [150] .
Ph Ph
Me H
N N
Co
MeO O O OMe
MeO
Br
(64) (65)
Cyclopropanation reactions can be promoted using copper or rhodium
catalysts or indeed systems based on other metals. As early as 1965 Nozaki
showed that chiral copper complexes could promote asymmetric addition of a
carbenoid species (derived from a diazoester) to an alkene. This pioneering
study was embroidered by Aratani and co-workers who showed a highly
enantioselective process could be obtained by modifying the chiral copper
Ph
H
Ph
i
+ N 2 CHCO 2 tBu
CO 2 tBu
H
80 % (inc. 4 % cis)
93 % ee
Scheme 50: Reagents and conditions: (i) Catalyst (65), room temperature, CH 2 Cl 2 , 24 h: