Page 54 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 54
the integration of biotransformations into catalyst 37
complex [151] . Subsequently many excellent metal-catalysed methods have been
developed for asymmetric cyclopropanation [152] , most being trans-selective for
the addition of diazo-ester to an alkene such as styrene: one example is shown
in Scheme 50 [153] . Only a few catalysts (for example a ruthenium±salen system)
have been found that promote asymmetric cyclopropanation to give cis-
products [154] . The range of asymmetric reactions of diazoesters has been
extended to additions to imines to furnish aziridine derivatives [155] .
Finally allylic substitution reactions involving, for example, replacement of
an acetate unit with a malonate residue (or other nucleophiles) has been re-
searched extensively by Trost and co-workers [156] . This group originally used
Pd(PPh 3 ) in the presence of a chiral phosphine to induce asymmetry but has
4
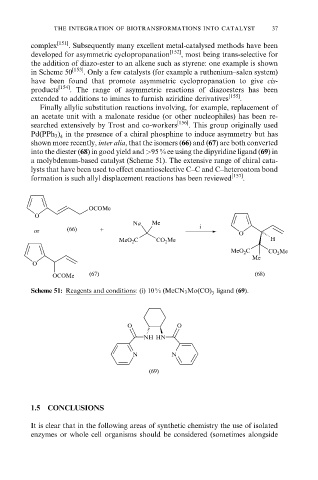
shown more recently, inter alia, that the isomers (66) and (67) are both converted
into the diester (68) in good yield and >95 % ee using the dipyridine ligand (69) in
a molybdenum-based catalyst (Scheme 51). The extensive range of chiral cata-
lysts that have been used to effect enantioselective C±C and C±heteroatom bond
formation is such allyl displacement reactions has been reviewed [157] .
OCOMe
O
Na Me i
or (66) + O
MeO 2 C CO 2 Me H
MeO 2 C CO 2 Me
Me
O
OCOMe (67) (68)
Scheme 51: Reagents and conditions: (i) 10 % (MeCN 3 Mo(CO) ligand (69).
3
O O
NH HN
N N
(69)
1.5 CONCLUSIONS
It is clear that in the following areas of synthetic chemistry the use of isolated
enzymes or whole cell organisms should be considered (sometimes alongside