Page 50 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 50
the integration of biotransformations into catalyst 33
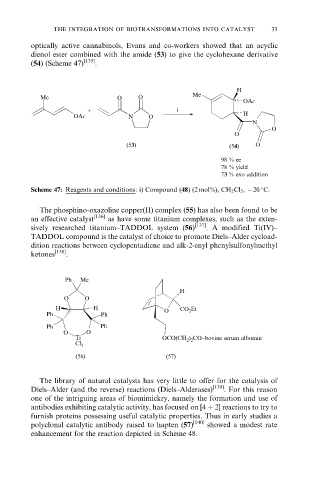
optically active cannabinols, Evans and co-workers showed that an acyclic
dienol ester combined with the amide (53) to give the cyclohexane derivative
(54) (Scheme 47) [135] .
H
Me O O Me
OAc
+ i
OAc N O H
N
O
O
(53) (54) O
98 % ee
78 % yield
73 % exo addition
Scheme 47: Reagents and conditions: i) Compound (48) (2 mol%), CH 2 Cl 2 , ÿ20 C.
The phosphino-oxazoline copper(II) complex (55) has also been found to be
an effective catalyst [136] as have some titanium complexes, such as the exten-
sively researched titanium±TADDOL system (56) [137] . A modified Ti(IV)±
TADDOL compound is the catalyst of choice to promote Diels±Alder cycload-
dition reactions between cyclopentadiene and alk-2-enyl phenylsulfonylmethyl
ketones [138] .
Ph Me
H
O O
H H CO 2 Et
Ph Ph O
Ph Ph
O O
Ti OCO(CH 2 ) 2 CO−bovine serum albumin
Cl 2
(56) (57)
The library of natural catalysts has very little to offer for the catalysis of
Diels±Alder (and the reverse) reactions (Diels±Alderases) [139] . For this reason
one of the intriguing areas of biomimickry, namely the formation and use of
antibodies exhibiting catalytic activity, has focused on [4 2] reactions to try to
furnish proteins possessing useful catalytic properties. Thus in early studies a
polyclonal catalytic antibody raised to hapten (57) [140] showed a modest rate
enhancement for the reaction depicted in Scheme 48.