Page 47 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 47
30 hydrolysis, oxidation and reduction
Me Me
N
OSiMe 3 O O
O O
Ti
O O N N
OMe
Bu t O H Cu 2+ H
CMe 3 X 2 CMe 3
(47): X = CF 3 SO 3 −
t Bu
(48): X = [SbF 6 ] −
(45) (46)
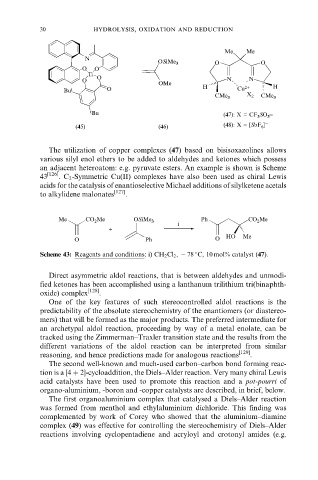
The utilization of copper complexes (47) based on bisisoxazolines allows
various silyl enol ethers to be added to aldehydes and ketones which possess
an adjacent heteroatom: e.g. pyruvate esters. An example is shown is Scheme
43 [126] . C 2 -Symmetric Cu(II) complexes have also been used as chiral Lewis
acids for the catalysis of enantioselective Michael additions of silylketene acetals
[127]
to alkylidene malonates .
Me CO 2 Me OSiMe 3 Ph CO 2 Me
i
+
O Ph O HO Me
Scheme 43: Reagents and conditions: i) CH 2 Cl 2 , ÿ 78 C, 10 mol% catalyst (47).
Direct asymmetric aldol reactions, that is between aldehydes and unmodi-
fied ketones has been accomplished using a lanthanum trilithium tri(binaphth-
oxide) complex [128] .
One of the key features of such stereocontrolled aldol reactions is the
predictability of the absolute stereochemistry of the enantiomers (or diastereo-
mers) that will be formed as the major products. The preferred intermediate for
an archetypal aldol reaction, proceeding by way of a metal enolate, can be
tracked using the Zimmerman±Traxler transition state and the results from the
different variations of the aldol reaction can be interpreted from similar
reasoning, and hence predictions made for analogous reactions [129] .
The second well-known and much-used carbon±carbon bond forming reac-
tion is a [4 2]-cycloaddition, the Diels±Alder reaction. Very many chiral Lewis
acid catalysts have been used to promote this reaction and a pot-pourri of
organo-aluminium, -boron and -copper catalysts are described, in brief, below.
The first organoaluminium complex that catalysed a Diels±Alder reaction
was formed from menthol and ethylaluminium dichloride. This finding was
complemented by work of Corey who showed that the aluminium±diamine
complex (49) was effective for controlling the stereochemistry of Diels±Alder
reactions involving cyclopentadiene and acryloyl and crotonyl amides (e.g.