Page 42 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 42
the integration of biotransformations into catalyst 25
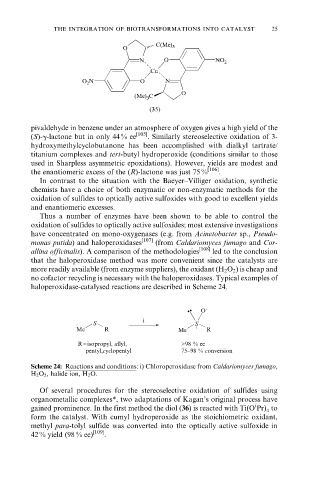
O C(Me) 3
N O NO 2
Cu
O 2 N O N
O
(Me) 3 C
(35)
pivaldehyde in benzene under an atmosphere of oxygen gives a high yield of the
(S)-g-lactone but in only 44 % ee [105] . Similarly stereoselective oxidation of 3-
hydroxymethylcyclobutanone has been accomplished with dialkyl tartrate/
titanium complexes and tert-butyl hydroperoxide (conditions similar to those
used in Sharpless asymmetric epoxidations). However, yields are modest and
the enantiomeric excess of the (R)-lactone was just 75 % [106] .
In contrast to the situation with the Baeyer±Villiger oxidation, synthetic
chemists have a choice of both enzymatic or non-enzymatic methods for the
oxidation of sulfides to optically active sulfoxides with good to excellent yields
and enantiomeric excesses.
Thus a number of enzymes have been shown to be able to control the
oxidation of sulfides to optically active sulfoxides; most extensive investigations
have concentrated on mono-oxygenases (e.g. from Acinetobacter sp., Pseudo-
monas putida) and haloperoxidases [107] (from Caldariomyces fumago and Cor-
allina officinalis). A comparison of the methodologies [108] led to the conclusion
that the haloperoxidase method was more convenient since the catalysts are
more readily available (from enzyme suppliers), the oxidant (H 2 O 2 ) is cheap and
no cofactor recycling is necessary with the haloperoxidases. Typical examples of
haloperoxidase-catalysed reactions are described in Scheme 24.
-
O
i
S S
Me R Me + R
R = isopropyl, allyl, >98 % ee
pentyl,cyclopentyl 75−98 % conversion
Scheme 24: Reactions and conditions: i) Chloroperoxidase from Caldariomyces fumago,
H 2 O 2 , halide ion, H 2 O.
Of several procedures for the stereoselective oxidation of sulfides using
organometallic complexes*, two adaptations of Kagan's original process have
i
gained prominence. In the first method the diol (36) is reacted with Ti(O Pr) to
4
form the catalyst. With cumyl hydroperoxide as the stoichiometric oxidant,
methyl para-tolyl sulfide was converted into the optically active sulfoxide in
42 % yield (98 % ee) [109] .