Page 38 - Catalysts for Fine Chemical Synthesis Vol 1 - Robert & Poignant
P. 38
the integration of biotransformations into catalyst 21
Similarly, racemic 1-trimethylsilyloct-(1E)-en-3-ol is epoxidized with
Ti(O ÿ i-Pr) , t-butylhydroperoxide and ()-di-isopropyl tartrate at ÿ20 8C to
4
give the epoxide (26) (> 99 % ee, 42 %) and recovered (R)-unsaturated alcohol [87] .
In general, when using ()-tartrates, the (S)-enantiomer of the allylic alcohol will
react faster.
The requirement for the presence of an adjacent alcohol group can be
regarded as quite a severe limitation to the substrate range undergoing asym-
metric epoxidation using the Katsuki±Sharpless method. To overcome this
limitation new chiral metal complexes have been discovered which catalyse the
epoxidation of nonfunctionalized alkenes. The work of Katsuki and Jacobsen in
this area has been extremely important. Their development of chiral manganese
(III)±salen complexes for asymmetric epoxidation of unfunctionalized olefins*
has been reviewed [88] .
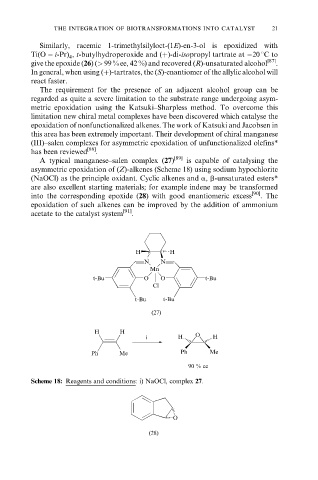
A typical manganese±salen complex (27) [89] is capable of catalysing the
asymmetric epoxidation of (Z)-alkenes (Scheme 18) using sodium hypochlorite
(NaOCl) as the principle oxidant. Cyclic alkenes and a, b-unsaturated esters*
are also excellent starting materials; for example indene may be transformed
into the corresponding epoxide (28) with good enantiomeric excess [90] . The
epoxidation of such alkenes can be improved by the addition of ammonium
acetate to the catalyst system [91] .
H H
N N
Mn
t-Bu O O t-Bu
Cl
t-Bu t-Bu
(27)
H H O
i H H
Ph Me Ph Me
90 % ee
Scheme 18: Reagents and conditions: i) NaOCl, complex 27.
O
(28)